Abstract
In this study we analyzed by immunohistochemistry the expression of TGF-β1 protein and TGF-β receptors I and II in 4 low-grade dysplastic nodules, 2 high-grade dysplastic nodules, 6 early, 22 small, and 62 advanced hepatocellular carcinomas. The expression of TGF-β1 protein by hepatocytes was decreased in advanced hepatocellular carcinoma compared with small or early hepatocellular carcinoma(P < .05). Frequent and intense staining of TGF-β1 protein was noted in the sinusoidal endothelium of advanced hepatocellular carcinomas despite of its decreased staining in hepatocellular carcinoma cells. Reduced expression of TGF-β receptors I and II compared with surrounding nontumorous tissue were noted from the early hepatocellular carcinoma stage suggesting that down-regulation of TGF-β receptors is correlated with progression from premalignant to malignant phenotype. Reduced expression of both TGF-β1 and TGF-β receptor II in neoplastic hepatocytes were also significantly correlated with increased tumor size and increased proliferative activity(P < .05). These findings suggest that during hepatocarcinogenesis, the inhibitory effects of TGF-β1 protein on hepatocellular carcinoma cells is outweighed by its effects on stromal elements, which, overall, contributes indirectly to a tumor growth stimulatory environment. Also, the growth-inhibitory effects of TGF-β1 may have been further negated by reduced TGF-β receptors on hepatocellular carcinoma cells.
Similar content being viewed by others
INTRODUCTION
TGF-β1 belongs to a family of polypeptides that play a central role in the regulation of cell growth and differentiation within both normal and transformed cells (1). In general, TGF-β1 inhibits the proliferation of normal epithelial cells including hepatocytes (2, 3, 4). However, the effects of TGF-β1 in tumor growth are more complex and controversial in that although they may directly inhibit proliferation of epithelial cancer cells, TGF-β1 may indirectly promote tumor growth by their angiogenic and immunosuppressive properties and effects in extracellular matrix (5, 6). TGF-β1 is known to generate these responses by interacting with cell surface receptors that are ubiquitously expressed: type I (55 kD), type II (80 kD), and type III (280 kD) receptors (7). Both TGF-β receptor I (TGF-β RI) and TGF-β receptor II (TGF-β RII) possess intrinsic serine-threonine kinase activity and signal through a heteromeric receptor complex (8, 9). TGF-β RI requires TGF-β RII to bind ligand whereas TGF-β RII requires the presence of TGF-β RI to signal (10). TGF-β1 RIII lacks consensus motif and does not directly participate in TGF-β1 signal transduction (11). Currently, the loss of functional TGF-β RI and/or RII expression is thought to play a critical role in the escape of tumor cells from TGF-β mediated cell cycle control and thus contribute to carcinogenesis (12, 13).
Likewise, previous studies have implicated the overexpression of TGF-β1 and reduced expression of TGF-β receptors in the development of hepatocellular carcinoma (14, 16). However, many of theses studies were carried out either in vitro or in animal models. The few in vivo studies that demonstrated TGF-β1 expression in human hepatocellular carcinoma tissue have been limited by the small number of cases precluding statistical significance (17, 18, 19). Moreover, no study has as yet evaluated the expression of TGF-β1 and TGF-β receptors in the premalignant stages of human hepatocellular carcinoma. In an attempt to clarify the roles of TGF-β1 and its receptors in multistage human hepatocarcinogenesis, we analyzed by immunohistochemistry the expression of TGF-β1 and TGF-β receptors I and II in a series of lesions including low-grade dysplastic nodules, high-grade dysplastic nodules, early hepatocellular carcinoma, small hepatocellular carcinoma and advanced hepatocellular carcinoma. We then investigated whether there was a correlationship between the expression of TGF-β1 and its receptors, and the many prognostic factors including tumor size, histologic grade, presence of vascular invasion, serum alpha-fetoprotein and proliferative index.
MATERIALS AND METHODS
A total of 96 cases of hepatocellular carcinoma and dysplastic nodules in surgically resected specimens were selected from the files of the department of surgical pathology of Yonsei University, College of Medicine.
All of the available hematoxylin and eosin-stained slides were reviewed for all cases. Dysplastic nodules were classified as low-grade or high-grade according to the criteria and nomenclature of International Working Party of the Terminology of Nodular Lesions of the Liver (20). Lesions with microfoci of hepatocellular carcinoma arising from dysplastic nodules were defined as early hepatocellular carcinoma (21, 22, 23). Lesions composed entirely of hepatocellular carcinoma without evidence of dysplastic nodules and measuring less than or equal to 3 cm were defined as small hepatocellular carcinoma (23), whereas those larger than 3 cm were defined as advanced hepatocellular carcinoma. The histologic grade of hepatocellular carcinoma was based on the Edmondson-Steiner classification.
Immunohistochemistry
Paraffin-embedded blocks containing sufficient amount of tumor as well as adjacent nontumorous tissue and showing minimal necrosis were chosen from each of the 96 cases. Immunohistochemical stains were performed with these paraffin blocks using a streptavidin-biotin complex immunoperoxidase technique. Primary antibodies used were directed against TGF-β1, TGF-β RI and TGF-β RII (Santa Cruz Biotechnology, Santa Cruz, CA) and Ki-67 (Novocastra, Newcastle upon Tyne, United Kingdom), all at a dilution of 1:100. The polyclonal antibodies detecting TGF-β1 was raised against the amino acids 328–352 at the carboxy terminus of the precursor form of TGF-β1 and non-cross-reactive with TGF-β2 or TGF-β3; TGF-β RI corresponding to the amino acids 158 to 179 of the precursor form of TGF-β RI, subtype of ALK5, and non-cross-reactive to TGF-β RII; and TGF-β RII corresponding to amino acids 246 to 266 of the precursor form of TGF-β RII and non-cross-reactive with TGF-β RIALK5 (Santa Cruz Biotechnology). The specificity of these antibodies were demonstrated previously (24, 25). Briefly, 4-micrometer sections of formalin-fixed, paraffin-embedded blocks were deparaffinized and hydrated in a series of xylene, graded alcohols, and water and finally washed in phosphate-buffered saline (PBS). The sections were immersed in a thermoresistant container filled with citrate buffer solution (pH 6.0) and placed in a pressure cooker and microwaved for 20 minutes. The sections were cooled at room temperature and rinsed with PBS. They were then incubated in 3% hydrogen peroxide for 30 minutes to block endogenous peroxidase activity and with normal bovine serum for another 30 minutes to reduce nonspecific antibody binding. The sections were then incubated overnight at 4°C with primary antibodies. After another PBS wash, the slides were further incubated with biotinylated rabbit anti-mouse antibody for 20 minutes at room temperature. The slides were washed again then reacted with avidin-biotin-peroxidase (DAKO, Carpenteria, CA) for 20 minutes followed by incubation with 3-amino-9-ethylcarbazole chromogen. The sections were finally counterstained with Mayer’s hematoxylin. To ensure antibody specificity, control slides were either incubated in the absence of primary antibody or with an unspecific IgG antibody. In both cases no immunostaining was detected.
Immunoreactivity and distribution pattern of TGF-β1, TGF-β RI, and TGF-β RII were examined in both the tumorous and adjacent nontumorous tissue. Immunoreactivity for TGF-β1, TGF-β RI, and TGF-β RII in hepatocytes were separately scored according to modification of a previously reported method (26, 27). This entails a three step categorization. The percentage of the positive cells were graded on a scale of 0 to 4+ (0: negative; 1+: 0–25%; 2+: 26–50%; 3+: 51–75%; 4+: 76–100%). Areas that showed positivity were further quantified from 0 to 3+ by the relative staining intensity (0: negative; 1+: weak; 2+: moderate; 3+: intense staining). The final score of 0 to 12 was obtained by multiplying the intensity and quantification measurements. The proliferative index (Ki-67 labeling index) was calculated by counting the number of Ki-67 positive hepatocytes over a total of 1000 hepatocytes under 400 × magnification.
Statistical Analysis
The results were expressed as mean ± standard deviation. For statistical analysis, the chi-squared test and one way ANOVA was used.
RESULTS
Clinicopathologic Features
A total of 96 cases of hepatocellular carcinoma and dysplastic nodules were included in this study: 4 low-grade dysplastic nodules (4.2%), 2 high-grade dysplastic nodules (2.1%), 6 early hepatocellular carcinoma (6.3%), and 84 hepatocellular carcinoma (87.5%). Of the 84 cases of hepatocellular carcinoma, 22 (22.9%) were small hepatocellular carcinoma and 62 (64.6%) were advanced hepatocellular carcinoma. The histologic grade of hepatocellular carcinoma was grade I in 14 cases (15.6%), grade II in 46 cases (51.1%), and grade III in 30 cases (33.3%). There were no grade IV hepatocellular carcinomas. The histologic grade of early hepatocellular carcinoma were all grade I.
In 95 of these cases, serologic viral markers were available revealing 70 (73.2%) HBsAg positive cases and 6 (6.3%) anti-HCV cases. Nineteen (20.0%) cases were negative for both HBsAg and anti-HCV. Cirrhosis was present in the adjacent nontumorous tissue in 43 cases (44.8%).
Expression Pattern of TGF-β1, TGF-β RI, and TGF-β RII in Nontumorous Tissue
TGF-β1, TGF-β RI, and TGF-β RII were variably expressed in many different types of cells in the nontumorous tissue by immunohistochemical stain.
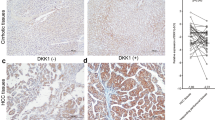
In the nontumorous tissue, hepatocytes at the periphery of cirrhotic nodules and near the border of the tumor showed frequent and intense cytoplasmic staining to TGF-β1, whereas periportal hepatocytes showed only occasional weak staining (Fig. 1A). Also, the epithelium of metaplastic bile ductules, especially those situated at the border of the tumor and present in active cirrhotic nodules showed intense cytoplasmic staining to TGF-β1. Interestingly, the endothelial cells lining the sinusoids of nontumorous tissue were generally negative to TGF-β1 in contrast to those lining the sinusoids of advanced hepatocellular carcinoma, which often displayed strong positivity (Fig. 2). The cytoplasm of macrophages were intensely stained with TGF-β1 but the fibroblasts and the fibrous stroma were negative.
Immunohistochemical stain for TGF-β1 in sinusoidal endothelium of cirrhotic nodule and hepatocellular carcinoma. A, Cirrhotic nodules shows diffuse positive reaction to TGF-β1 in the cytoplasm of hepatocytes but negative reaction in the sinusoidal endothelium. B, Hepatocellular carcinoma shows reverse pattern of TGF-β1 staining; negative reaction in the tumor cells and strong positive reaction in the sinusoidal endothelium (LSAB method, A: × 200, B: × 40).
TGF-β RI demonstrated diffuse, cytoplasmic staining in the nonneoplastic hepatocytes. The bile ductules, bile ducts, vascular smooth muscle wall and macrophages were also positive to TGF-β RI but the extent and intensity of staining were generally lower as compared with that of TGF-β RII. The fibroblasts were occasionally positive to TGF-β RI.
TGF-β RII showed diffuse and moderately intense staining in the nonneoplastic hepatocytes. Similar to TGF-β1, TGF-β RII was most intensely stained in the hepatocytes situated along the periphery of the cirrhotic nodule and near the border of the tumor (Fig. 1B). The epithelium of metaplastic bile ductules were similarly intensely stained with TGF-β RII (Fig. 1C). Fibroblasts were occasionally weakly positive to TGF-β RII but the fibrous stroma was always negative.
Positive Rates of TGF-β1, TGF-β RI, and TGF-β RII Expression in Tumor
The expression rates of TGF-β1, TGF-β RI, and TGF-β RII in the tumors are summarized in Table 1. Only those cases showing immunoreactivity in the hepatocytes comprising the tumor were considered to be positive. All three antibodies showed a cytoplasmic staining pattern in the preneoplastic (Fig. 3) and neoplastic hepatocytes (Fig. 4). Of a total of 96 cases, TGF-β1 and TGF-β RI expression were observed in 53 (55.2%) and 77 (80.2%) cases, respectively. TGF-β RII was expressed in all the lesions (100%). All cases of dysplastic nodules, both low-grade and high-grade as well as early hepatocellular carcinoma universally expressed TGF-β1, TGF-β RI, and TGF-β RII. However, small and advanced hepatocellular carcinoma showed decreased TGF-β1 and TGF-β RI positive rates: 63.6% and 72.7% in small hepatocellular carcinoma, and 43.5% and 79.0% in advanced hepatocellular carcinoma, respectively.
Immunohistochemical stain for TGF-β1, TGF-β RI, and TGF-β RII in hepatocellular carcinoma. A, TGF-β1 is focally expressed among hepatocellular carcinoma cells. B, Note diffuse positive staining of TGF-β RI in adjacent normal hepatocytes as compared with negative staining in hepatocellular carcinoma. C, Reduced expression of TGF-β RII in hepatocellular carcinoma (LSAB method, A: × 100, B: × 40, C: × 200).
Staining Scores of TGF-β1, TGF-β RI, and TGF-β RII in Tumor
The expression levels of TGF-β1, TGF-β RI, and TGF-β RII in the various stages of hepatocarcinogenesis were compared by measuring their average staining scores. The average staining scores for TGF-β1 in the advanced hepatocellular carcinoma (1.5 ± 2.16) were significantly lower as compared with that of small hepatocellular carcinoma (3.4 ± 3.79) (P < .05). TGF-β1 was most abundantly expressed in the low-grade dysplastic nodules (9.5 ± 3.00). In high-grade dysplastic nodules, the level of TGF-β1 expression (5.0 ± 4.24) was similar to that of early hepatocellular carcinoma (5.3 ± 2.07).
There was no significant difference in the mean staining scores for TGF-β RI in the various stages of hepatocarcinogenesis (Table 2). However, the mean staining scores of TGF-β RI in early hepatocellular carcinoma (2.8 ± 1.83), small hepatocellular carcinoma (2.9 ± 3.37) and advanced hepatocellular carcinoma (4.1 ± 3.26) revealed to be consistently and significantly lower as compared with that of corresponding nontumorous tissue (6.7 ± 1.63, 9.0 ± 3.34, 8.4 ± 3.47), respectively (P < .05) (Table 2).
The mean staining scores for TGF-β RII showed a tendency to decrease with tumor progression (Table 2), measuring highest in the low-grade dysplastic nodule (9.0 ± 2.0) and lowest in the advanced hepatocellular carcinoma (6.2 ± 2.59). The mean staining scores for TGF-β RII in advanced hepatocellular carcinoma (6.2 ± 2.59) was significantly lower than that of small hepatocellular carcinoma (8.1 ± 2.84). As in the case of TGF-β RI, the mean staining scores for TGF-β RII in early, small and advanced hepatocellular carcinoma were significantly lower than that of corresponding nontumorous tissue (Table 2). But there was no difference in the mean staining scores for TGF-β RII in the low- and high-grade dysplastic nodules when compared with their corresponding nontumorous tissue (Table 2).
Correlation of TGF-β1, TGF-β1 RI, and TGF-β1 RII Expression with Tumor Size, Vascular Invasion, Serum Alpha-fetoprotein, and Histologic Grade
The expression levels of TGF-β1, TGF-β RI, and TGF-β RII in tumor correlated with variable clinicopathologic factors are summarized in Table 3. TGF-β1 expression was inversely correlated with tumor size and histologic grade (P < .05). Furthermore, decreased TGF-β1 expression was associated with the presence of vascular invasion and increased serum alpha-fetoprotein levels. However, these findings did not achieve statistical significance.
TGF-β RI expression was inversely correlated with serum alpha-fetoprotein(P < .05). However, there was no significant correlation between TGF-β RI expression and tumor size, histologic grade and vascular invasion.
Significant reduction in TGF-β RII expression was found with increased tumor size (P < .05). TGF-β RII also tend to decrease with increase in serum alpha-fetoprotein levels and advancing histologic aggressiveness.
Correlation of TGF-β1, TGF-β RI, and TGF-β RII Expression with Proliferative Index
The proliferative index (Ki-67 score) in various stages of hepatocarcinogenesis are shown in Fig. 5. The levels of TGF-β1 and TGF-β RII expression in tumor was inversely correlated with the Ki-67 score (P < .05) (Fig. 6, Fig. 7). But there was no significant correlation between TGF-β RI expression and the Ki-67 score.
DISCUSSION
TGF-β1 is an extensively studied cytokine with diverse biologic activities including regulation of cell growth and differentiation, control of extracellular matrix formation, angiogenesis and immunomodulation (28, 29). Recent studies reveal that TGF-β1 is also involved in the development and progression of cancer and contrary to its well-known inhibitory effects on normal epithelial cells, elevated levels of TGF-β1 mRNA have been reported in many cancers such as liver, lung, breast, kidney and prostate (14, 15). Furthermore, TGF-β1 expression has been associated with increased cell proliferation in gastric and thyroid cancers (27, 30) and with disease progression in breast and prostate cancer (31, 32).
Studies in human gastric, breast and prostate cancers have claimed the cancer cells as the source of increased TGF-β1 and the overexpression of TGF-β1 protein in these cancer cells have been confirmed by immunohistochemical stains (27, 31, 32). However, the source and site of TGF-β1 expression in liver is still controversial. It is well established that TGF-β1 is expressed in nonparenchymal cells among which Kupffer cells, sinusoidal cells and transforming rat and human hepatic stellate cells have been identified as the source of elevated TGF-β1 mRNA and TGF-β1 protein in diseased human and rat liver (33, 34). Until recently, the vast majority of studies do not point to hepatocytes as cellular sources of TGF-β1 under normal, inflammatory and fibrotic conditions. However, more recent studies have not only identified TGF-β1 mRNA and protein in the hepatocytes of long-term-phenobarbital-treated rats but also displayed that injured rat hepatocytes are capable of releasing latent TGF-β1 during liver regeneration suggesting hepatocytes as a major source of TGF-β1 (35, 36). Regarding human liver tissue, some authors have reported hepatocellular carcinoma cells and their perineoplastic stroma to be immunopositive to TGF-β1 (17, 37), whereas Sue et al. have reported an absence of TGF-β1 immunostaining in hepatocellular carcinoma cells (18).
In the present study using immunohistochemical staining, TGF-β1 expression was detected in the tumor cells in 53 of the 96 (55.2%) cases of hepatocellular carcinoma and dysplastic nodules. The adjacent nonneoplastic hepatocytes showed positivity to TGF-β1 as well and was typically most intensely stained in the regenerating hepatocytes located near the border of the tumor and along the periphery of cirrhotic nodules. Similar intense staining was also observed in the epithelium of metaplastic bile ductules. Interestingly, the accentuated TGF-β1 staining in these areas colocalized with that of TGF-β RII. TGF-β1 was never stained in the stroma. These immunohistochemical results are contradictory to two previous reports. In the first one, Abou-Shady et al. reported intense TGF-β1 immunostaining in the hepatocellular carcinoma cells and the perineoplastic stroma whereas no or mild immunostaining was present in normal liver (37). Similarly, the second report by Bedossa et al. stated that only the neoplastic hepatocytes were immunoreactive to TGF-β1, whereas normal and cirrhotic hepatocytes were entirely negative (17). Moreover, in their study, TGF-β1 was more frequently stained in the stromal tissue of both the cirrhotic nodules and hepatocellular carcinoma rather than the parenchymal cells. The reason for this discrepancy in immunoreactivity we speculate is due to the difference in TGF-β1 antibody used. The TGF-β1 antibody used in Bedossa’s study seems to react predominantly with the extracellular form of TGF-β1, whereas the antibody we and perhaps Sue et al. have used is similar to the LC anti-TGF-β1 antisera (38), which reacts predominantly with the intracellular forms of TGF-β1. The preferential expression of TGF-β1 and TGF-β RII in the regenerating hepatocytes located along the tumor border, periphery of cirrhotic nodules, and metaplastic bile ductules in the non-neoplastic tissue is in accordance with previous studies that indicated TGF-β1 protein expressed by the regenerating hepatocytes is involved in preventing uncontrolled hepatocyte proliferation (3, 4). In another report, TGF-β1 RNA transcripts have been localized by in situ hybridization to some hepatocytes in the periphery of regenerating nodules in cirrhotic human liver suggesting that hepatocytes may well be capable of expressing the TGF-β1 gene even under non-neoplastic circumstances and that TGF-β1 may influence parenchymal cell growth in distinct areas of lobule by negative autocrine regulatory loops (39).
Although there is general agreement about the overexpression of TGF-β1 mRNA in hepatocellular carcinoma, relatively few studies have examined TGF-β1 protein expression in human hepatocellular carcinomas. Ours is the first to our knowledge to attempt to compare and characterize TGF-β1 expression in a large series of hepatocellular carcinomas including the premalignant stages. By comparing the immunohistochemical scores of TGF-β1 in various stages of hepatocellular carcinoma, we found that the expression of TGF-β1 protein by tumor cells was significantly decreased in advanced hepatocellular carcinoma as compared with small or early hepatocellular carcinoma. Also, TGF-β1 expression in the cancer cells was inversely correlated with tumor size, proliferative index and histologic grade. Furthermore, there was a tendency for reduced TGF-β1 expression in hepatocellular carcinomas showing vascular invasion and increased serum alpha-fetoprotein. Overall, reduced TGF-β1 protein expression in the neoplastic hepatocytes was associated with increased aggressiveness of tumor. A comparable study by Yamaguchi et al. demonstrated that TGF-β1 expression in tumor was inversely correlated with PCNA labeling index and tumor size (40). Furuta et al. also indicated that immunohistochemically TGF-β1 was normally expressed by cancer cells of well differentiated hepatocellular carcinoma, but the expression was weak or negative in poorly differentiated type (41). Yuen et al. demonstrated that patients with hepatocellular carcinoma and patients with cirrhosis of variable causes all had significantly higher serum levels of total TGF-β1 compared with those of controls (42). However, a lower serum total TGF-β1 level was related to an increased tumor size, which is similar to the findings of Yamaguchi et al. Yuen et al. suggested that this may be another adaptive mechanism by which the hepatocellular carcinoma minimizes the TGF-β1-induced apoptotic effect on the tumor cells when TGF-β1 level exceeds the tumor tolerable levels (42).
Alternatively, we suggest that the reasons for the discrepancy between endogenously increased TGF-β1 levels in hepatocellular carcinoma tissue and decreased expression of TGF-β1 protein by neoplastic hepatocytes in advanced hepatocellular carcinoma observed in our study may be due to the discordance between the main sites of TGF-β1 synthesis; the neoplastic hepatocytes, and the main sites of action; perineoplastic stroma. Thus, although there is no doubt about increased production of TGF-β1 by the hepatocellular carcinoma cells, the actual effects of increased TGF-β1 protein may be predominantly realized in surrounding stromal elements by a paracrine mechanism. This is in keeping with published data that tumor cells lose the ability to interact with the TGF-β1 protein in an autocrine fashion, and instead indirectly stimulate their growth by the paracrine action of TGF-β1 on supporting stromal elements, which include suppression of immune response and enhancement of both angiogenesis and function of connective tissue. In support of this theory, we found in our study that despite of decreased TGF-β1 protein expression by the neoplastic hepatocytes, the endothelial cells lining the sinusoids of advanced hepatocellular carcinoma were often intensely stained. Interestingly, neither the dysplastic nodules nor the cirrhotic nodules showed this pattern of TGF-β1 staining in the sinusoidal endothelial cells. Factor et al. also underscored the paracrine effects of TGF-β1 during tumor progression by demonstrating that although the expression of TGF-β1 transgene was found to be reduced or absent in tumorous relative to nontumorous hepatic tissue, endogenous TGF-β1 mRNA levels were markedly elevated in most of the tumors appearing in TGF-β1 and c-myc/TGF-β1 transgenic mice (43).
However, further serial studies also utilizing antibodies that react with extracellular forms of TGF-β1 or better still, discriminate active and latent TGF-β1 may provide further insights to the relationship between the expression of TGF-β1 and tumor progression in the present series of patients.
On the other hand, it also has been suggested that for most cancers, expression of TGF-β1 ligand does not correlate with malignancy, whereas loss of growth inhibitory response to TGF-β1 at the cellular level is probably a more important step in malignant progression, and TGF-β1 receptors and proteins involved in signaling by TGF-β1 appears to act as tumor suppressors (44).
In our study, using antibodies that recognize the cytoplasmic domain of the receptors (45, 46, 47), TGF-β RI expression was observed in 80.2% (77 of 96) of cases of hepatocellular carcinoma and dysplastic nodules, whereas TGF-β RII expression was observed in all cases (100%). However, semiquantitative analysis of the immunohistochemical reactions showed that the mean staining scores of TGF-β RI and RII in early, small and advanced hepatocellular carcinoma but not dysplastic nodules were significantly lower than that of surrounding nontumorous tissue, suggesting that the reduced expression of TGF-β receptors is correlated with development of hepatocellular carcinoma. A previous study has similarly shown that the degree of TGF-β RII immunolocalization in hepatocellular carcinoma liver tissue was significantly decreased compared with those in chronic hepatitis or liver cirrhosis (45). Moreover, they demonstrated by western blot analysis that 50% (3 of 6) of the patients showed an obvious decrease of the TGF-β RII protein in hepatocellular carcinoma tissue compared with that in non-hepatocellular carcinoma tissue. In the hepatocellular carcinoma tissues in which TGF-β RII expression was not decreased, they presumed other factors that induce hepatocarcinogenesis may be involved (48, 49). Sue et al., showed a significant decrease of M6-P/IGF-IIr (mannose-6-phosphate/insulin-like growth factor receptor), TGF-β RI, and TGF-β RII at both the mRNA and protein levels in hepatocellular carcinomas compared with surrounding liver. They suggested that the decreased level of TGF-β1 protein in the malignant epithelial cells of hepatocellular carcinoma may result from the decreased expression of both the M6-P/IGF-IIr and TGF-β receptors (18). Taken together, these data indicate that at least some cases of hepatocellular carcinoma has reduced expression of TGF-β receptors for TGF-β. This may provide a selective growth advantage to hepatocellular carcinoma by escaping the growth-inhibitory signals of the endogenously increased TGF-β1 and may be linked with critical steps in hepatocarcinogenesis.
Our study showed that the immunoreactivity of TGF-β RII in advanced hepatocellular carcinoma was significantly decreased compared with small or early hepatocellular carcinoma. In addition, tumoral TGF-β RII immunoreactivity correlated inversely with both the tumor size and the tumor proliferative index, suggesting that down-regulation of TGF-β RII could lead to enhanced tumor cell proliferation and therefore support tumor progression.
By analogy with colon carcinoma, mutations in one of the two microsatellites within the TGF-β RII coding region may be responsible for the reduced TGF-β receptor immunoreactivity in hepatocellular carcinoma (50). In support of this theory, Furuta et al. demonstrated increasing frequency of inactivating mutations of the TGF-β RII with more aggressive hepatocellular carcinomas (41). However, not all reports support this conclusion (51, 52) and in those cases without TGF-β RII gene mutation, altered regulation of transcription may negatively influence the stability or function of the protein. On the other hand, a reduction in the detection of TGF-β RII by immunohistochemistry could also result if the receptor existed in a form other than its known functional state, influenced by post-transitional modifications or substrate bound conformational changes that diminish antibody activity.
In conclusion, using immunohistochemistry, we showed that the TGF-β1 protein may be expressed in a wider variety of hepatocytes than previously reported, including regenerating hepatocytes, dysplastic hepatocytes and hepatocellular carcinoma cells. Furthermore, the reduced expression of TGF-β RII in the hepatocellular carcinoma cells was correlated with accelerated tumor growth and increasing malignancy, and the expression of TGF-β1 protein by hepatocellular carcinoma cells was noted to decrease in the course of high-grade progression. Additional functional and molecular studies are needed to investigate the etiology of a possible receptor defect. Whether variation in the intensity of immunohistochemical staining of the TGF-β RII or TGF-β1 provides independent prognostic clinical information will be the subject of future analysis.
References
Lanprecht SA, Schwartz B, Glickman A . TGF-β1 in intestinal epithelial differentiation and neoplasia. Anticancer Res 1989; 9: 1877–1882.
Roberts AB, Anzano MA, Wakefield LM, Roche NS, Stern DF, Sporn MB . Type βtransforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci USA 1985; 82: 119–128.
Nakamura T, Tomita Y, Hirari R, Yamaoka K, Kaji K, Ichihara A . Inhibitory effect of TGF-β on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun 1985; 133: 1042–1050.
Strain AJ, Frazer A, Hill DJ, Milner RD . TGF-β inhibits DNA. synthesis in hepatocytes isolated from normal and regenerating rat liver. Biochem Biophys Res Commun 1987; 145: 436–442.
Roberts AB, Thompson NL, Heine U, Flanders C, Sporn MB . Transforming growth factor-β. Possible roles in carcinogenesis. Br J Cancer 1988; 57: 594–600.
Arrick BA, Lopez AR, Elfman F, Ebner R, Damsky CH, Derynck R . Altered metabolic and adhesive properties and increased tumorigenesis associated with increased expression of transforming growth factor-beta1. J Cell Biol 1992; 118: 715–726.
Massague J . Receptors for the TGF-β family. Cell 1992; 69: 1067–1070.
Cheiftz S, Bassols A, Stanley K, Ohata M, Greenberger J, Massague J . Heterodimeric transforming growth factor-beta. Biological properties and interaction with three types of cell receptors. J Biol Chem 1988; 263: 10783–10789.
Franzen P, ten Dijke P, Ichijo H, Yamashita H . Cloning of a TGF-β type I receptor that forms a heteromeric complex with the TGF-β type II receptor. Cell 1994; 75: 681–692.
Wrana JL, Attisano L, Weiser R, Ventura F, Massague J . Mechanism of activation of the TGF-β receptor. Nature 1994; 370: 341–347.
Lopez-Casillas F, Cheifetz S, Doody J, Andres JL, Lane WS, Massague J . Structure and expression of membrane proteoglycan betaglycan, a component of the TGF-β receptor. Cell 1991; 75: 681–692.
Mulder KM, Segarini PR, Morris SL, Ziman JM, Choi HG . Role of receptor complexes in resistance or sensitivity to growth inhibition by TGF-β in intestinal epithelial cell clones. J Cell Physiol 1993; 154: 162–174.
Guo Y, Jacobs SC, Kyprianou N . Down-regulation of protein and mRNA expression for transforming growth factor-β (TGF-β) type I and type II receptors in human prostate cancer. Int J Cancer 1997; 71: 573–579.
Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Willams RD, Bringman TS, et al. Synthesis of mRNAs for TGFs alpha and beta and the EGF receptor by human tumors. Cancer Res 1987; 47: 707–712.
Ito N, Kawata S, Tamura S, Takishi K, Shirari Y, Kiso S, et al. Elevated levels of TGF-β1 mRNA and its polypeptide in human hepatocellular carcinoma. Cancer Res 1991; 51: 4080–4086.
Reisenbichler H, Chari R, Boyer I, Jirtle RL . Transforming growth factor-β receptors type I, II, and III in phenobarbital-promoted rat liver tumors. Carcinogenesis (London) 1994; 15: 2763–2767.
Bedossa P, Pelter E, Terris B, Franco D, Poynard T . TGF-β1 and TGF-β1 receptors in normal, cirrhotic and neoplastic human livers. Hepatology 1995; 21: 760–766.
Sue SR, Chari RS, Kong FM . Transforming growth factor beta receptors and mannose-6-phosphate/insulin-like growth factor II receptor expression in human hepatocellular carcinoma. Annu Surg 1995; 222: 171–178.
Orsatti G, Hytiroglou P, Thung SN, Ishak KG, Paronetto F . Lamellar fibrosis in the fibrolamellar variant of hepatocellular carcinoma: a role for transforming growth factor β. Liver 1997; 17: 152–156.
International Working Party. Terminology of nodular hepatocellular lesions. Hepatology 1995; 25: 983–993.
Sakmoto M, Hirohashi S, Shimosato Y . Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol 1991; 22: 172–178.
Okuda K . Hepatocellular carcinoma: recent progress. Hepatology 1992; 15: 948–963.
Kanai T, Hirohashi S, Upton MP, Noguchi M, Kishi K, Makuuchi M, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer 1987; 60: 810–819.
Jakowlew SB, Kondaiah PK, Flanders KC, Thompson NL, Dillard PJ, Sporn MB, et al. Increased coordinate expression of growth factor mRNAs accompanies viral transformation of rodent cells. Oncogene Res 1988; 2: 135–148.
Jakowlew SB, Moody TW, Mariano JM . Transforming growth factor-beta receptors in human cell lines: analysis of transcript, protein and proliferation. Anticancer Res 1997; 17: 1849–1860.
Shimizu M, Saitoh Y, Itoh H . Immunohistochemical staining of H-ras oncogene product in normal, benign and malignant human pancreatic tissues. Hum Pathol 1990; 21: 607–612.
Hirayama D, Fujimori T, Satonaka K, Nakamura T, Kitazawa S, Horio M, et al. Immunohistochemical study of epidermal growth factor and transforming growth factor-β in the penetrating type of early gastric cancer. Hum Pathol 1992; 23: 681–685.
Roberts AB, Sporn MB . The transforming growth factor-β. In: Sporn MB, Roberts AB, editors. Peptide growth factors and their receptors. Heidelberg: Springer-Verlag; 1990. pp. 419–472.
Ruscetti FW, Palladino MA . Transforming growth factor β and the immune system. Prog Growth Factor Res 1991; 53: 159–175.
Jasani B, Wyllie FS, Wright PA, Lemoine NR, Williams ED, Wynford-Thomas D . Immunocytochemically detectable TGF-β is associated with malignancy in thyroid epithelial neoplasia. Growth Factors 1990; 2: 149–155.
Gorsch SM, Meomoli VA, Stukel TA, Gold LI, Arrick BA . Immunohistochemical staining for transforming growth factor β1 associates with disease progression in human breast cancer. Cancer Res 1992; 52: 6949–6952.
Eastham JA, Truong LD, Rogers E, Kattan M, Flanders KC, Scardino PT, et al. Transforming growth factor-β1: comparative immunohistochemical localization in human primary and metastatic prostate cancer. Lab Invest 1995; 73: 628–635.
Braun L, Mead JE, Panzica M, Mikumo R, Bell GI, et al. TGF-β mRNA increases during liver regeneration. A possible paracrine mechanism of growth regulation. Proc Natl Acad Sci USA 1988; 85: 1539–1543.
Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thogeirsson SS . Cellular distribution of transforming growth factor-beta and procollagen types I, II and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest 1990; 85: 1833–1843.
Nakatsukasa H, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS . Expression of TGF-β1 during chemical hepatocarcinogenesis in the rat. Lab Invest 1991; 65: 511–517.
Bissel DM, Wang SS, Jarnagin WR, Roll FJ . Cell specific expression of TGF-β in rat liver-evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest 1995; 96: 447–455.
Abou-Shady M, Baer HU, Friess H, Berberat P, Zimmermann A, Graber H, et al. Transforming growth factor betas and their signaling receptors in human hepatocellular carcinoma. Am J Surg 1999; 177: 209–215.
Flanders KC, Thompson NL, Cissel DS . Transforming growth factor-β1 histochemical localization with antibodies to different epitopes. J Cell Biol 1989; 108: 653–660.
Milani S, Herbst H, Shuppan D, Stein H, Surrenti C . Transforming growth factor β1 and β2 are differentially expressed in fibrotic liver disease. Am J Pathol 1991; 139: 1221–1229.
Yamaguchi M, Yu L, Hishikawa Y, Yamanoi A, Kubota H, Nagasue N . Growth kinetic study of human hepatocellular carcinoma using proliferating cell nuclear antigen and Lewis Y Antigen: Their correlation with transforming growth factor-α and β1. Oncology 1997; 54: 245–251.
Furuta K, Misao S, Kiyoshi T, Tagaya T, Fukuzawa Y, Ishikawa T, et al. Gene mutation of transforming growth factor β1 type II receptor in hepatocellular carcinoma. Int J Cancer 1999; 81: 851–853.
Yuen MF, Norris S, Evans LW, Langley PG, Hughes RD . Transforming growth factor-beta 1, activin and follistatin in patients with hepatocellular carcinoma and patients with alcoholic cirrhosis. Scand J Gastroenterol 2002; 2: 233–238.
Factor VM, Kao CY, Santoni-Rugiu E, Woitach JT, Jensen MR, Thorgeirsson SS . Constitutive expression of mature transforming growth factor β1 in the liver accelerates hepatocarcinogenesis in transgenic mice. Cancer Res 1997; 57: 2089–2095.
Serra R, Moses HL . Tumor suppressor genes in the TGF-β signaling pathway? Nature Med 1996; 2: 390–391.
Ueno T, Hashimoto O, Kimura R, Torimura T, Kawaguchi T, Nakamura T, et al. Relation of type II Transforming growth factor-β receptor to hepatic fibrosis and hepatocellular carcinoma. Int J Oncol 2001; 18: 49–55.
Roy SK, Kole AR . Transforming growth factor-β receptor type II expression in the hamster ovary: cellular sites, biochemical properties, and hormonal regulation. Endocrinology 1995; 136: 4610–4620.
Lazzereschi D, Ranieri A, Mincione G, Taccgna S, Nardi F, Colletta G . Human malignant thyroid tumors displayed reduced levels of transforming growth factor β receptor type II messenger RNA and protein. Cancer Res 1997; 57: 2071–2076.
Fukuda K, Ma X, Kren BT, Steer CJ . Altered regulation of Src tyrosine kinase by transforming growth factor beta 1 in human hepatoma cell line. Hepatology 1998; 28: 764–804.
Tanaka S, Wanda JR . Insulin receptor substrate I overexpression in human hepatocellular carcinoma cells prevents transforming growth factor beta 1-induced apoptosis. Cancer Res 1996; 56: 3391–3394.
Markowitz S, Wang J, Myeroff L, Parsons R, Scen L, Luterbaugh J, et al. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 1995; 268: 1336–1338.
Kawate S, Takenoshita S, Ohwada S, Mogi A, Fukusato T, Makita F, et al. Mutation analysis of the transforming growth factor beta type II receptor, Smad2, and Smad4 in hepatocellular carcinoma. Int J Oncol 1999; 14: 127–131.
Vincent F, Hagiwara K, Ke Y, Stoner GD, Demetrick DJ, Bennett WP . Mutation analysis of the transforming growth factor beta type II receptor in sporadic human cancers of the pancreas, liver, and breast. Biochem Biophys Res Commun 1996; 223: 561–564.
Acknowledgements
This paper was supported by the Yonsei University College of Medicine Research Fellow Fund and Brian Korea 21 Project for Medical Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paik, S., Park, Y., Kim, H. et al. Expression of Transforming Growth Factor-β1 and Transforming Growth Factor-β Receptors in Hepatocellular Carcinoma and Dysplastic Nodules. Mod Pathol 16, 86–96 (2003). https://doi.org/10.1097/01.MP.0000047308.03300.9C
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000047308.03300.9C
Keywords
This article is cited by
-
Overexpression of FOXG1 contributes to TGF-β resistance through inhibition of p21WAF1/CIP1 expression in ovarian cancer
British Journal of Cancer (2009)
-
TGFbeta induces apoptosis and EMT in primary mouse hepatocytes independently of p53, p21 Cip1 or Rbstatus
BMC Cancer (2008)
-
Deficiency of G1 regulators P53, P21Cip1and/or pRb decreases hepatocyte sensitivity to TGFβ cell cycle arrest
BMC Cancer (2007)
-
DDX3, a DEAD box RNA helicase, is deregulated in hepatitis virus-associated hepatocellular carcinoma and is involved in cell growth control
Oncogene (2006)
-
Involvement of programmed cell death 4 in transforming growth factor-β1-induced apoptosis in human hepatocellular carcinoma
Oncogene (2006)