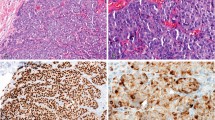
Abstract
There has been considerable variability in the reported results of immunohistochemical staining for some diagnostically relevant antigens. Our objectives in this study were to (1) use a multitumor tissue microarray with tissue from 351 cases received in our department, representing 16 normal tissues and 47 different tumor types, to compare immunohistochemical staining results in our laboratory with published data, using a panel of 22 antibodies; (2) assess interlaboratory variability of immunohistochemical staining for S-100 using this microarray; and (3) test the ability of hierarchical clustering analysis to group tumors by primary site, based on their immunostaining profile. Tissue microarrays consisting of duplicate 0.6-mm cores from blocks identified in the hospital archives were constructed and stained according to our usual protocols. Antibodies directed against the following antigens were used: B72.3, bcl-2, carcinoembryonic antigen, c-kit, pankeratin, CD 68, CD 99, CK 5/6, CK 7, CK 8/18, CK19, CK 20, CK 22, epithelial membrane antigen, estrogen receptor, melan-A, p53, placental alkaline phosphatase, S-100, synaptophysin, thyroid transcription factor-1, and vimentin. Staining results on the array cases were compared with published results, and hierarchical clustering analysis was performed based on the immunohistochemical staining results. Unstained slides of the multitumor tissue microarray were sent to five other diagnostic immunohistochemistry laboratories and stained for S-100 protein. The staining results from the different laboratories were compared. Staining results using our current methods and samples from our laboratory were compatible with those described in the literature for most antigens. Placental alkaline phosphatase staining was not specific with our protocol, showing staining of a broad spectrum of different tumors; this finding initiated a review of our recent requests for placental alkaline phosphatase immunostaining and revealed two instances in which placental alkaline phosphatase positivity was incorrectly interpreted as evidence of a germ cell tumor. S-100 staining was less sensitive but more specific for the diagnosis of melanoma or neural tumor in our laboratory, compared to some published reports. Assessment of interlaboratory variability of S-100 immunostaining showed that there was more frequent staining of carcinomas in some laboratories, resulting in decreased specificity of S-100 staining in distinguishing melanoma from carcinoma. Hierarchical clustering analysis showed a strong trend for tumors to cluster by tissue of origin, but there were significant exceptions. We conclude that multiple-tumor microarrays are an efficient method for assessing the sensitivity and specificity of staining with any antibody used diagnostically. As a tool for quality assurance, they offer the advantage of taking into account local differences in tissue fixation, processing, and staining. They also allow cost-effective assessment of interlaboratory variability in immunohistochemical staining. Results of hierarchical clustering analysis show the potential for panels of immunohistochemical stains to identify the primary site of metastatic carcinomas but also confirm the limitations of currently available antibodies in giving unequivocal tissue-specific staining patterns.
Similar content being viewed by others
INTRODUCTION
Pathologists rely heavily on published data regarding immunohistochemical staining profiles of antibodies introduced into diagnostic immunohistochemistry laboratories, as there is typically only limited on-site validation of the sensitivity and specificity of antibody staining. Local variations in tissue fixation, antigen retrieval, and staining methods may significantly affect the staining profile obtained with a given antibody (1, 2), and it is not clear that results found in the literature consistently can be generalized to individual diagnostic immunohistochemistry laboratories. Kononen et al. (3) described a technique for the evaluation of a large number of formalin-fixed, paraffin-embedded samples on a single slide through the creation of tissue microarrays. We hypothesized that the application of tissue microarray technology would facilitate quality assurance in the diagnostic immunohistochemistry laboratory, and the three objectives of this study were to (1) compare the results of immunohistochemical staining of tissue processed in our laboratory with published data, using a panel of 22 antibodies of diagnostic utility and a large series of tumors and normal tissues, representing multiple primary sites and cell lineages; (2) compare staining for S-100 in multiple laboratories, using the same set of cases, to assess interlaboratory variability in staining; and (3) test the ability of hierarchical clustering analysis to group tumors according to primary site, based on their immunophenotype, with a panel of 22 antibodies.
MATERIALS AND METHODS
Three hundred fifty-one cases representing 63 different normal tissues and tumor types were selected from the Vancouver Hospital and Health Science Center’s clinical database of cases for the years 2000 and 2001. Selected case slides and paraffin-fixed materials were retrieved from the hospital archives. All tissues were fixed in neutral buffered 10% formalin. Specific paraffin blocks containing tumor or normal tissues were identified, and areas containing the tissue of interest were marked on the paraffin block with a marker.
Tissue microarrays comprising duplicate 0.6-mm cores from the selected blocks were constructed using a Beecher Instruments Micro Tissue Arrayer as previously described (4). Four-micrometer-thick sections were cut from completed array blocks and transferred to silanized glass slides. Sections from these arrays were then stained with hematoxylin and eosin to assess adequacy. Further sections were stained with the panel of antibodies listed in Table 1 using a Ventana (Tucson, AZ) automated immunohistochemical stainer according to the manufacturer’s guidelines. These antibodies were chosen based on their frequency of use in our laboratory, with particular attention to inclusion of antibodies used in cases in which there is a question about the cell lineage or primary site of a tumor. Antigen retrieval was done as indicated in Table 1. Microwave antigen retrieval consisted of placing the slides in 10 mm citrate buffer (pH 6.0) in a pressure cooker (Nordic Ware) and microwaving on high power until the buffer had boiled under pressure for 4 minutes. At this point, microwaving was stopped and the slides were incubated in the pressure cooker for a further 20 minutes, after which they were removed and rinsed. Proteinase antigen retrieval consisted of a 4-minute incubation in protease-1 solution (Ventana) according to the supplier’s recommended protocol.
Sections stained with the various antibodies were then scored using a 4-point scale on which 0 = negative (<5% of cells staining), 1 = uninterpretable (e.g. because of loss of the tissue or excessive background staining), 2 = weak staining (i.e. 5–50% of cells showing weak to intermediate intensity staining in an appropriate subcellular distribution), 3 = strong staining (i.e. ≥5% of cells showing very intense staining or >50% of cells showing weak to moderately intense staining, in an appropriate subcellular distribution). Scores were entered into a Microsoft Excel spreadsheet. Scoring results were simplified into either negative (score of 0) or positive (score of 2 or 3) categories, and uninterpretable results were eliminated from further consideration (i.e. they were not used for calculations of sensitivity and specificity). Score results for duplicate cores were consolidated into one score with positive staining always superceding a negative or uninterpretable result.
Individual cores on the tissue microarray that did not have interpretable staining results for >80% of the antibodies used in our panel were also excluded from the analysis.
Staining results on our array samples were compared with those in the literature using the ImmunoQuery Website (www.immunoquery.com), with the permission of the creator, Dr. D. Frisman, supplemented by searches of the National Medical Library’s PubMed Website.
Unstained sections of the tissue microarray were submitted to five diagnostic immunohistochemistry laboratories (designated A–E) and stained for S-100 protein. S-100 was chosen because it has been routinely used for many years, allowing staining protocols to become standardized, and, although newer melanoma markers have come into use, S-100 remains widely used for diagnostic purposes. These laboratories stained the slides according to their usual protocol and returned the slides to be scored by the same pathologists who scored the other cases presented in this study, using the same scoring system described previously. Laboratories B and E used polyclonal anti S-100 antibody from DAKO, at a dilution of 1:400, with no pretreatment and protease pretreatment for antigen retrieval, respectively. Laboratories A, C, and D used the same anti-S-100 antibody described in Table 1, at dilutions of 1:1600, 1:4000, and 1:4000, respectively. Laboratory A used heat pretreatment for antigen retrieval, whereas Laboratories C and D used protease pretreatment.
Hierarchal clustering analysis of our tissue microarray data was performed using software tools that originally were designed for analyzing cDNA microarray data (5). An Excel macro, TMA-Deconvoluter (6), was designed and written specifically for processing of raw tissue microarray staining data into a format that is compatible for use with the previously developed Cluster software (5). The clustered data were then graphically viewed using TreeView (Cluster and TreeView software are freely available programs that can be accessed at http://rana.lbl.gov/EisenSoftware.htm).
RESULTS
The staining patterns observed on our multiple-tumor tissue microarray were in agreement with published data in a large majority of cases in which published data exist, and our complete set of staining results can be viewed at www.pathology.ubc.ca/immuno/Table2.xls. For example, the results from our panel of cytokeratin (CK) subset antibodies were concordant with published sensitivities (Table 2). We further noted that the CK subset staining results for a variety of tumors represented on our array have not previously been well characterized. CK 5/6 stained the basal cells of the prostatic acini without staining the luminal cells or cells of prostatic adenocarcinoma, a pattern of staining previously described for the anti-cytokeratin antibody 34 beta E12, which has been studied extensively as a diagnostic adjunct for the diagnosis of prostatic adenocarcinoma (7, 8, 9).
A notable exception among our panel of IHC stains assayed was the PLAP antibody. The results generated using the standard staining protocol at our institution were found to be nonspecific, staining almost all tissue cores present on the array, and to differ significantly from the sensitivity and specificity profile reported in the literature. When we reviewed recent cases in which PLAP staining had been requested, we identified two cases of mediastinal tumors that were initially thought to be germ cell tumors based on PLAP immunoreactivity. In both cases, the correct diagnosis of lymphoma was arrived at based on an extended panel of immunohistochemical markers. In contrast, the c-kit stain was noted to have a high sensitivity (10/11 = 91%) and relatively high specificity (218/256 = 85%) in distinguishing germ cell tumors from carcinomas. This is in agreement with the reported sensitivity and specificity profiles for c-kit (10) and supports its role as a useful stain for the identification of germ cell tumors.
Comparison of our S-100 staining results with those in published data was problematic because of the wide range of reported results (Table 3). This variability has been attributed to differences in the interpretation of staining results. We required both nuclear and cytoplasmic immunoreactivity to consider there to be positive staining, whereas some studies have accepted cytoplasmic staining only as evidence of positivity. Using our more stringent evaluation criteria, which are currently accepted by most investigators (1), we found that S-100 immunoreactivity was seen less frequently in epithelial malignancies than previously has been reported (e.g. 0/19 breast carcinomas stained positively, compared with 21/48 in the literature, as identified through ImmunoQuery) and is a sensitive (5/5, 100%) and specific (232/251, 92%) marker of melanocytic versus epithelial differentiation.
Comparison of staining for S-100 at six different laboratories showed generally concordant results, with staining of most or all cases of malignant melanoma by each laboratory (Table 4). There was some variability in the frequency with which nonmelanoma tumors stained positively for S-100, with Laboratory B showing a higher rate of positivity in these cases (for example, 50% of renal cell carcinomas, 36.4% of ovarian carcinomas, and 60% of thyroid carcinomas were positive for S-100 on the tissue microarray stained in their laboratory). The reason for this variability is not readily apparent. Although Laboratory B used a different antibody than did most of the participating laboratories, Laboratory E used the same antibody without a similar frequency of staining of nonmelanoma tumors.
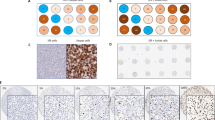
Hierarchal clustering analysis of the staining patterns generated with our panel of antibodies was able to successfully group many cases by their clinical diagnosis (Fig. 1). Use of clustering analysis in this setting illustrates its potential for identifying tumors of unknown origin. In most cases, tumors from the same primary site cluster together, based on the relatedness of the immunoprofile (Fig. 1B–C). There were, however, significant individual discrepant results, with, for example, individual cases of lung, anal, ovarian, and uterine carcinoma clustering together based on a similar nonspecific carcinoma immunophenotype (Fig. 1D).
Graphical representation of the hierarchical cluster analysis. Positive staining is indicated by a red cube, absence of staining as green, and no available data as gray, for each of the antibodies indicated at the top of the figure. All the data are shown (A) and can be viewed in expanded form at www.pathology.ubc.ca/immuno/Figure1.GIF. Subsets of these data show the tendency for tumors to cluster according to site of origin (B and C), although some tumors from different sites do cluster together (D).
DISCUSSION
Immunohistochemical staining has become an indispensable adjunct in the practice of surgical pathology. Despite the widespread use of immunohistochemistry, there remain significant problems with quality assurance; variability in tissue fixation, processing, staining methodologies and reagents, and interpretation of staining results persists. When new antibodies are introduced into a laboratory, there is a period during which optimization of staining occurs through titration, determination of antigen retrieval technique, and staining of a series of archival cases. Most antibodies in use diagnostically detect antigens that show a limited range of expression and are therefore cell type specific and, to a lesser extent, tissue specific in their staining patterns. The extent of the initial workup of antibodies being introduced into the laboratory, particularly with respect to the range of different tissues and tumors studied, has been limited by costs (both labor and reagents). Thus, there has been a reliance on published data on staining sensitivity and specificity that may not be applicable in a given laboratory.
Tissue microarrays of the sort used in this study were first described by Kononen et al. (3) and represent a significant advance over earlier multitissue blocks (e.g. sausage blocks) in terms of the number of samples and uniformity of sample size. Their utility in external quality assurance programs has been suggested (11), and we have demonstrated how a breast tumor array was useful in assessing inter- and intralaboratory reproducibility of staining for estrogen receptor (4). The results of staining two or more 0.6-mm cores have been shown to be representative of staining of whole sections (4, 12, 13, 14). We sought to test the utility of multiple-tumor microarrays in intralaboratory quality control of an extended diagnostic panel of antibodies and in interlaboratory quality control for a single antibody.
We were able to identify a significant problem with lack of sensitivity with one of the antibodies on our diagnostic menu. Because PLAP staining is used relatively infrequently, no single pathologist had accrued sufficient expertise to identify the nonspecific PLAP staining. Use of the multiple-tumor microarray allowed rapid identification of this problem. Use of the multitumor array also allowed us to define the specificity of S-100 staining in our laboratory. S-100 immunostaining showed a greater degree of specificity than has been reported in some previous studies, and our findings can now be taken into account in interpreting staining results with this antibody in our laboratory. We anticipate using the multiple-tumor microarray regularly to assess the performance of antibodies currently in use, as well as for testing new batches of antibody received in the laboratory and new antibodies we are considering introducing into practice. The multiple-tumor microarray is particularly useful for this latter application because it would be impossible to stain 351 slides with each new antibody to determine sensitivity and specificity of staining, whereas a single multiple-tumor microarray slide is readily stained. We have made the results of our multiple-tumor microarray staining available to users of our laboratory in tabular form (may be viewed at www.pathology.ubc.ca/immuno/Table2.xls) as an aid to their rational ordering and interpretation of immunohistochemical stains. In an assessment of interlaboratory variability, we found that there was variation in the frequency with which nonmelanoma tumors stain positively for S-100. These results are of clinical importance because S-100 staining is less discriminatory in the differential diagnosis of melanoma versus carcinoma with increasing frequency of staining of cases of carcinoma, and knowledge of this information can influence interpretation of S-100 staining results.
Our approach also has potential for discovery. The reported immunoprofile with some of the antibodies we use is not comprehensive; we observed immunoreactivity for melan-A in occasional prostatic adenocarcinomas, for example. A useful observation was of CK5/6 staining of prostatic basal epithelial cells. Although the pattern is identical to that reported with the 34 beta E12 antibody, the staining for CK5/6 is, in our laboratory, more intense, without background staining, and thus easier to interpret. Subsequent use of CK5/6 staining in atypical glandular lesions and low-grade prostatic adenocarcinomas has supported the utility of this stain as an adjunct in this difficult differential diagnosis. Subsequent to our performing the studies described herein, a large study on CK 5/6 immunostaining has appeared (15). The results of this study are summarized in Table 2, together with results of earlier studies cited in ImmunoQuery. Chu and Weiss (15) also noted the staining of prostatic basal epithelial cells for CK 5/6, and the results of our study with a tissue microarray are indistinguishable from their results obtained with conventional whole-section immunohistochemistry, supporting the validity of our approach. Even a superficial estimate of the costs of staining multiple sections, compared with tissue microarrays, makes apparent the potential savings that can be realized through tissue microarrays. If one estimates a reagent cost of $10 perslide, reagents for our study cost $20, compared with approximately $5000 for the study of Chu and Weiss (15), with these studies generating identical results. Although there are labor costs associated with construction of the tissue microarrays, they are offset by reduced labor costs associated with tissue sectioning and staining.
A significant problem in the interpretation of immunohistochemical staining results occurs when there are apparent inconsistencies in the staining profile (for example, a pleural tumor that is calretinin, Ber-EP4, CEA, and B72.3 positive, and negative for WT-1 and CK5/6). Although a typical immunophenotypic profile is readily interpretable, we have, at present, no good system for dealing with complex tumor immunoprofiles. With extended panels of antibodies applied to difficult cases, there is an increased likelihood that there will be apparent inconsistencies in the staining profiles generated, with one or more antibodies giving “aberrant” staining results. We have at present no systematic approach to interpretation of an extended data set generated by staining a case with a large panel of antibodies. Clustering analysis has been applied successfully to gene expression data, based on the expression of thousands of genes, and has been remarkably successful in its ability to group tumors according to their primary site, as well as in subgrouping tumors that are not reproducibly subclassifiable based on conventional morphology (16, 17, 18, 19). We have shown that clustering analysis with a panel of 22 antibodies can successfully group tumors according to their primary site, but there remain significant overlaps between groups. It should be noted that the dynamic range of immunostaining scores is significantly narrower than that obtained for mRNA levels in gene microarray experiments and that as a result, the clustering of tumors based on immunoreactivity can be expected to be less well defined than is seen in gene expression array studies. Nonetheless, hierarchical clustering analysis holds great promise for the immunohistochemical subclassification of tumors according to primary site, although our study illustrates the need for better, more tissue-specific, antigen targets. The emerging gene expression data will undoubtedly facilitate identification of appropriate antigens.
CONCLUSION
In summary, multiple-tumor tissue arrays are an efficient method of assessing the sensitivity and specificity of antibodies used in determining origin or cell type of human tumors. The results from the panel of immunohistochemical stains run on our arrays allowed us to generate staining profiles for the various antibodies that are specific to the fixation and staining procedures at our institution. The power of this type of quality assurance analysis allowed us to quickly detect antibodies that were not performing as expected. Multitumor microarrays also allow cost-effective assessment of interlaboratory variation in immunohistochemical staining.
References
Seidal T, Balaton AJ, Battifora H . Interpretation and quantification of immunostains. Am J Surg Pathol 2001; 25: 1204–1207.
Wick MR, Mills SE . Consensual interpretive guidelines for diagnostic immunohistochemistry. Am J Surg Pathol 2001; 25: 1208–1210.
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, et al. Tissue microarray for high-throughput molecular profiling of tumor specimens. Nat Med 1998; 4: 844–847.
Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, Akbari M, et al. Assessment of inter-laboratory variation in the immunohistochemical determination of estrogen receptor using a breast cancer tissue microarray Am J Clin Pathol (in press).
Eisen MB, Spellman PT, Brown PO, Botstein D . Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 1998; 95: 14863–16868.
Liu CL, Prapong W, Natkuman Y, Alizadeh A, Montgomery K, Gilks CB, et al. Software tools for high-throughput analysis and archiving of immunohistochemistry staining data obtained with tissue microarrays. Am J Pathol (in press).
Green R, Epstein JI . Use of intervening unstained slides for immunohistochemical stains for high molecular weight cytokeratin on prostate needle biopsies. Am J Surg Pathol 1999; 23: 567–570.
Goldstein NS, Underhill J, Roszka J, Neill JS . Cytokeratin 34 beta E-12 immunoreactivity in benign prostatic acini. Quantitation, pattern assessment, and electron microscopic study. Am J Clin Pathol 1999; 112: 69–74.
Iczkowski KA, Cheng L, Crawford BG, Bostwick DG . Steam heat with an EDTA buffer and protease digestion optimizes immunohistochemical expression of basal cell-specific antikeratin 34betaE12 to discriminate cancer in prostatic epithelium. Mod Pathol 1999; 12: 1–4.
Izquierdo MA, Van der Valk P, Van Ark-Otte J, Rubio G, Germa-Lluch JR, Ueda R, et al. Differential expression of the c-kit proto-oncogene in germ cell tumours. J Pathol 1995; 177: 253–258.
Moch H, Kononen J, Kallioniemi OP, Sauter G . Tissue microarrays: what will they bring to molecular and anatomic pathology? Adv Anat Pathol 2001; 8: 14–20.
Camp RL, Charette LA, Rimm DL . Validation of tissue microarray technology in breast carcinoma. Lab Invest 2000; 80: 1943–1949.
Nocito A, Bubendorf NA, Maria Tinner E, Suess K, Wagner U, Forster T, et al. Microarrays of bladder cancer tissue are highly representative of proliferative index and histological grade. J Pathol 2001; 194: 349–357.
Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 2001; 159: 2249–2256.
Chu PG, Weiss LM . Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol 2002; 15: 6–10.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403: 503–511.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747–752.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001; 98: 10869–10874.
Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 1999; 286: 531–537.
Acknowledgements
We acknowledge Dr. D. Frisman for kindly allowing us to use his ImmunoQuery Website to rapidly assess published immunostaining data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, F., Nielsen, T., Alkushi, A. et al. Tissue Microarrays Are an Effective Quality Assurance Tool for Diagnostic Immunohistochemistry. Mod Pathol 15, 1374–1380 (2002). https://doi.org/10.1097/01.MP.0000039571.02827.CE
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000039571.02827.CE
Keywords
This article is cited by
-
A Microfluidic Immunostaining System Enables Quality Assured and Standardized Immunohistochemical Biomarker Analysis
Scientific Reports (2017)
-
Clinical and prognostic value of MET gene copy number gain and chromosome 7 polysomy in primary colorectal cancer patients
Tumor Biology (2015)
-
HER3 protein expression in relation to HER2 positivity in patients with primary colorectal cancer: clinical relevance and prognostic value
Virchows Archiv (2015)
-
Combined epithelial-mesenchymal transition with cancer stem cell-like marker as predictors of recurrence after radical resection for gastric cancer
World Journal of Surgical Oncology (2014)
-
Double-positive expression of high-mobility group box 1 and vascular endothelial growth factor C indicates a poorer prognosis in gastric cancer patients
World Journal of Surgical Oncology (2013)