Abstract
Microsatellite instability (MSI) is a hallmark of the DNA mismatch repair deficiency that is one of the pathways of gastric carcinogenesis. Clinicopathologic characteristics of MSI+ gastric cancers remain unclear. To determine the correlation between MSI status and clinical features, we analyzed 327 consecutive gastric cancers for the occurrence of MSI in the BAT-26 marker. Because it has been proven that MSI at BAT-26 reflects the MSI+ phenotype, cancers with alteration at BAT-26 were categorized as having the MSI+ phenotype. The expressions of hMLH1, hMSH2, p53, MUC1, MUC2, and CEA were evaluated immunohistochemically using the tissue array method. The MSI+ phenotype was found in 9.5% (31/327) of gastric cancers examined. MSI+ gastric cancers were significantly associated with older age, antral location, Borrmann's gross Type II, intestinal subtype, lower prevalence of lymph node metastasis, and lower pTNM stage (P < .05). By multivariate logistic regression, MSI+ gastric cancers had a lower prevalence of lymph node metastasis independent of tumor invasion (P < .001). MSI+ gastric cancers displayed frequent frameshift mutations of transforming growth factor-β type II receptor (90.3%), BAX (61.3%), hMSH3 (38.7%), and E2F4 (61.3%) genes and diminished hMLH1 (24/31) or hMSH2 (4/31) expressions. The MSI+ phenotype correlated with patient survival in advanced gastric carcinoma (P = .046). In conclusion, MSI+ phenotype in gastric cancers was found to have distinct clinicopathologic characteristics and to be predictive of a favorable outcome in advanced carcinoma.
Similar content being viewed by others
INTRODUCTION
Although the incidence rate of gastric cancer has been declining steadily, gastric cancer remains the second most common malignant tumor in the world (1, 2) and contributes to significant cancer mortality, particularly in Asia (China, Japan, and Korea) and parts of Europe and Latin America. Multiple environmental factors, including Helicobacter pylori infection (3) and dietary factors (4), have been implicated in the initiation of gastric carcinogenesis. Although much has been learned recently about the molecular genetic alterations associated with the development of gastric cancers, much about them still has remained unclear.
Microsatellite instability (MSI) is a form of genomic instability associated with defective DNA mismatch repair in tumors (5). The majority of cancers of the hereditary nonpolyposis colon cancer (HNPCC) syndrome (6) and about 15% of unselected colorectal cancers have MSI+ phenotype (7). Clinicopathologic characteristics of MSI+ colorectal cancers are proximal location, younger age, lower lymph node metastasis, and a better survival rate (7, 8). The stomach is a frequent site of extracolonic cancer development in patients with HNPCC (9) and is one of the organs in which primary sporadic tumors show MSI+ phenotype (10, 11, 12, 13, 14, 15). Defects of the mismatch repair system and MSI play an important role in early stage of gastric carcinogenesis. In the adenoma–carcinoma sequence of the stomach, gastric adenoma had a high frequency of MSI, and it persisted after malignant transformation (16). To determine the correlations between MSI status and clinicopathologic variables affecting the prognosis and survival of gastric cancer patients, we analyzed 327 consecutive cases.
MATERIALS AND METHODS
Patients and Samples
Surgically resected gastric cancer cases were examined from the files of the Department of Pathology, Seoul National University College of Medicine that were kept between January 1, 1995, and June 30, 1995. In total, 352 patients were treated at the hospital and 336 specimens (95%) were available for the study. The age, sex, tumor location, gross type according to Borrmann's classification, tumor size, lymphatic invasion, and pTNM stage (17) were evaluated by reviewing medical charts and pathologic records. The mean age of the patients was 54.4 years, and 93.3% of patients underwent curative resection (R0 according to the UICC guideline). The study included 223 advanced gastric carcinomas and 113 early gastric carcinomas. Mean diameter of tumor was 6.04 ± 2.96 cm in advanced gastric carcinomas and 3.46 ± 2.51 cm in early gastric carcinomas (P < .001). No patient had fulfilled the Amsterdam criteria for HNPCC (18). No patient had received preoperative chemo- or radiotherapy. Glass slides were reviewed to determine the histologic type (according to the World Health Organization and Lauren's classifications). The clinical outcome of patients was followed from the date of surgery to the date of death or to December 1, 1999. The follow-up period was 1 60 months (mean, 42 mo). The cases lost to follow-up and to deaths from any other causes than gastric cancer were regarded as censored data during the analysis of survival rates.
Microsatellite Analysis
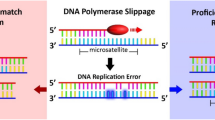
The DNA of cancerous tissue and corresponding normal gastric mucosa from 327 of 336 patients with consecutive gastric cancers was obtained from formalin-fixed, paraffin embedded surgical blocks. The DNA was extracted by proteinase K digestion and the phenol–chloroform procedures. The extracted DNA was amplified by PCR with fluorescent dye–labeled primers on two mononucleotide repeat microsatellite markers, BAT-26 and BAT-25 (located within intron 5 of the hMSH2 gene and introns of the c-kit oncogene, respectively). DNA was detected by a temperature-controlled DNA Sequencer (PRISM 377, Perkin-Elmer Corp., Foster City, CA), and fragment analyses were carried out with Genscan software (Perkin-Elmer). MSI status was determined by size variation and the occurrence of additional bands in the PCR product from tumor DNA and not observed in DNA from normal tissue from the same patient (19). Because previous reports and investigations have proven that MSI at BAT-26 reflects an MSI+ phenotype (10, 15, 16, 20, 21, 22), cancers with alteration at BAT-26 were categorized as having an MSI+ phenotype (Fig. 1). In this study, no short BAT-26 allele was found in PCR products from corresponding normal DNA.
Frameshift Mutation Analysis
To detect frameshift mutations of the coding regions in MSI+ gastric cancers, the repetitive mononucleotide tracts of the following genes were amplified using primers as previously described (23, 24): poly(A)10 tract of transforming growth factor-β type II receptor (TGFβRII), poly(G)8 tract of insulin-like growth factor II receptor (IGFIIR), poly(G)8 tract of BAX, poly(C)8 tract of hMSH6, poly(A)8 tract of hMSH3, and poly(AGC)13 tract of E2F4 genes. The reaction involved 32 cycles at 94° C for 1 minute, 53 to 60° C for 1 minute, and 72° C for 1 minute. The presence of additional bands in the PCR products from tumor DNA, not observed in the DNA of normal tissue, was counted as frameshift mutations.
Immunohistochemistry
Core tissue biopsies (2 mm in diameter) were taken from individual paraffin-embedded gastric tumors (donor blocks) and arranged in a new recipient paraffin block (tissue array block) using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea). Each tissue array block contained ≤60 cases, with a total of 336 cases in six array blocks. An adequate case was defined as a tumor occupying >10% of core area. As an internal control, each block contained normal gastric mucosa from body, antrum, and intestinal metaplasia. Four-μm sections were cut from each tissue array block, deparaffinized, and dehydrated.
Immunohistochemical staining against hMLH1 (dilution 1:50, Clone G168-728, 1μg/mL; Pharmingen, San Diego, CA), hMSH2 (1:100, Clone FE11, 0.5 μg/mL; Oncogene Science, Cambridge, MA), p53 protein (1:100, mouse monoclonal antibody DO7: DAKO, Carpinteria, CA), carcinoembryonic antigen (CEA; 1:100, mouse monoclonal antibody, DAKO), glycosylated MUC1 (1:100, mouse monoclonal antibody NCL-MUC-1: Novocastra Laboratories), and MUC2 (1:100, mouse monoclonal antibody NCL-MUC-2: Novocastra Laboratories) was performed using a streptavidin-biotin-peroxidase complex method (labeled streptavidin–biotin) after an antigen retrieval process using microwaves (three times for 5 min each) for hMSH2, p53, CEA, MUC1, and MUC2 and using an autoclave for hMLH1. CEA and p53 protein were not expressed in the normal mucosa of all slides, but hMLH1 and hMSH2 were expressed in the normal mucosa. MUC1 was weakly expressed in foveolar epithelium of antrum, and MUC2 was expressed in intestinal metaplasia. For statistical analysis, the results of MUC1 and MUC2 immunostaining were considered to be positive if >20% of the neoplastic cells were stained. When ≥10% of cancer cells showed strong cytoplasmic staining, CEA protein expression was considered to be positive. Those cases with ≥10% of nuclear staining in the tumor cells were considered to overexpress the p53 protein. When <10% of cancer cells showed nuclear staining, we considered the case to be an hMLH1 or hMSH2 expression loss (Fig. 2).
Microscopic features of immunohistochemistry. MSI+ gastric cancer showed complete loss of hMLH1 protein, but adjacent lymphoid cells and stromal cells are positive for the protein (A, 200 ×). In another MSI+ gastric cancer diminished hMSH2 protein expression were found (B, 200 ×), but adjacent normal mucosa expressed hMSH2 protein. Immunostaining of p53 (C, 200 ×), CEA (D, 100 ×), and MUC1 (E, 200 ×) and MUC2 (F, 200 ×) proteins revealed positive expression pattern.
Statistical Analyses
The chi-square test or Fisher's exact test (two-sided) was performed to determine the correlation between MSI+ phenotype and the clinicopathologic parameters. The association between MSI status and regional lymph node metastasis was evaluated with multivariate logistic regression. Survival curves were estimated using the Kaplan-Meier product-limit method, and the significance of differences between the survival curves was determined using the log-rank test. Multivariate survival analysis was performed using the Cox proportional hazards model. The results were considered to be statistically significant when P values were <.05. All statistical analyses were conducted using the SPSS version 9.0 statistical software program (SPSS, Chicago, IL).
RESULTS
Clinicopathologic Characteristics of MSI+ Gastric Cancers
Of the 327 tested specimens of consecutive gastric cancers, 31 (9.5%) had microsatellite instability. Three of 31 MSI+ gastric cancers did not show instability at BAT-25, but there was no gastric cancer with instability at BAT-25 but not at BAT-26. Table 1 shows the correlation between clinicopathologic parameters and MSI+ phenotype. Gastric cancers with MSI+ phenotype were characterized by older age (P = .009), antral location (P = .022), Type II according to Borrmann's gross classification (P = .001), and intestinal type according to Lauren's classification (P = .01). MSI+ cancers were more likely to be well differentiated or moderately differentiated adenocarcinomas than were MSI− cancers, but this was without statistical significance. Little difference was noted between lymphoid infiltration of MSI+ cancers (2/29, 6%) and that of MSI− cancers (12/269, 4%). Gastric cancers with associated gastric adenoma had a significantly higher frequency of MSI+ phenotype (3/6) than did gastric cancers without gastric adenoma (28/321, P < .05).
MSI+ gastric cancers were significantly associated with lower lymph node metastasis (P = .004) and lower pTNM stage (P = .017). By multivariate logistic regression, regardless of the depth of tumor invasion, gastric cancers with MSI+ phenotype had a lower prevalence of lymph node metastasis (P < .001, Table 2). However, no correlation was found between MSI+ phenotype and depth of tumor invasion or distant-organ metastasis.
Frameshift Mutations in the Coding Regions
Frameshift mutations in the coding regions of target genes were studied, and mutations were found in 90.3% (28/31) for TGFβRII, 9.7% (3/31) for IGFIIR, 61.3% (19/31) for BAX, 22.6% (7/31) for hMSH6, 38.7% (12/31) for hMSH3, and 61.3% (19/31) for E2F4 genes (Table 3). The mutation of the TGFβRII gene showed the greatest correlation with MSI+ phenotype. Three of 31 MSI+ gastric cancers that did not show instability at BAT-25 had frameshift mutations of the coding regions (Table 3).
Immunohistochemical Staining of Consecutive Gastric Cancers
To understand the origin of MSI, we examined the expression of hMLH1 and hMSH2 proteins. Of the 31 MSI+ gastric cancers, 24 cancers and 4 cancers showed markedly diminished hMLH1 and hMSH2 expressions in tumor cell nuclei, respectively. Four cancers of 235 MSI− gastric cancers also showed negative hMLH1 expression, and none of 277 MSI− gastric cancers showed negative hMSH2 expression.
Immunostaining of p53, CEA, MUC1 and MUC2 proteins was performed using the tissue array method (Table 4). The frequency of p53 protein expressions was found to be slightly lower in MSI+ cancers (7/30, 23%) than in MSI− cancers (99/277, 36%) but this was without statistical significance (P = .226). Cytoplasmic expressions of CEA were found less frequently in MSI+ gastric cancers (8/29, 27.6%) than in MSI− cancers (157/275, 57.0%; P = .008). Fourteen (47.0%) of 30 MSI+ cancers expressed MUC2 mucin, whereas 71 (26.0%) of 274 MSI− cancers did (P = .030).
Survival Analysis
The survival of patients with MSI+ phenotype (5-y survival rate, 85.4 ± 6.7%) was better than that of patients with MSI− phenotype (5-y survival rate, 71.8 ± 6.7%), but this was without statistical significance (P = .120). By Kaplan-Meier survival curves stratified according to disease progression (advanced and early carcinomas), MSI+ phenotype was significantly correlated with patient survival in advanced carcinoma (P = .046, Table 5, Fig. 3). Frameshift mutations of TGFβRII had a survival advantage in advanced carcinoma (P = .025), but frameshift mutations of other target genes were not associated with overall survival (IGFIIR, P = .228; BAX, P = .332; hMSH6, P = .523; hMSH3, P = .129; E2F4, P = .282). In patients who were 65 years of age or younger, a survival advantage associated with the MSI+ phenotype was found (P = .039). In terms of other clinicopathologic parameters, tumor location, Lauren's classification, lymphatic invasion, and pTNM stage were found to be significantly correlated with patient survival (P < .05). By multivariate Cox regression model, pTNM stage was found to be significantly and independently associated with patient survival (P < .001), but MSI+ phenotype (P = .484) and Lauren's classification (P = .253) were not independently associated with patient survival. MSI+ phenotype did not show a significant survival advantage in patients with advanced gastric carcinoma (P = .350) or patients who were 65 years of age or younger (P = .154) independently of pTNM stage and Lauren's classification.
Kaplan-Meier survival curves for patients with stomach cancer (+: censored cases). A, pTNM stage were significantly correlated with patient survival (P < .001). B, MSI+ phenotype was significantly associated with patient survival in advanced gastric carcinoma (AGC; P = .046). C, MSI+ phenotype was significantly associated with patient survival in patients 65 years or younger (P = .039).
DISCUSSION
Carcinogenesis is a long-term, multistep process driven by multiple genetic and epigenetic changes in the susceptible cells, which gain a selective growth advantage and undergo clonal expansion (25). Genetic instability is an important factor in the rapid accumulation of these genetic changes (26). MSI+ phenotype was found in 10 to 45% of gastric cancers in previous studies, depending on the group of cases studied and the definition of MSI (11, 12, 13, 14, 15). In this study, we evaluated MSI status in consecutive gastric cancers, and 31 of 327 gastric cancers (9.5%) showed MSI+ phenotype.
Cancers from different mutational pathways are thought to have different clinical features. For example, MSI+ colorectal cancers were found to have distinct clinicopathologic characteristics in a population-based series (7). However, the clinicopathologic characteristics of MSI+ gastric cancers were inconclusive because of smaller or potentially biased studies (27, 28, 29). We have found different clinical features associated with MSI+ gastric cancers. In our large and consecutive series, MSI+ gastric cancer was characterized by older age, antral location, Borrmann's gross Type II, intestinal type, the lower prevalence of lymph node metastasis, and a lower pTNM stage.
The incidence of small and early gastric cancer is higher in Asia and undoubtedly has been increased by early diagnosis (30). The overall 5-year survival rate of patients with gastric cancers was 73.6% in this study: 63.0% in advanced gastric carcinoma and 95.7% in early carcinoma (data not shown). The MSI+ phenotype did not show a survival advantage in patients with early carcinoma (P > .05), probably because of their high survival rate. We stratified survival curves according to disease progression (early versus advanced carcinoma), and MSI+ phenotype was significantly correlated with patient survival in advanced carcinoma (P = .046). However, it was controversial whether MSI+ gastric cancers had independent survival advantage or not. By multivariate Cox regression model, MSI+ phenotype was not independently associated with patient survival. MSI+ phenotype was significantly associated with the lower prevalence of lymph node metastasis regardless of the depth of tumor invasion, but no correlation between MSI+ phenotype and depth of tumor invasion or distant-organ metastasis was found. Therefore, MSI+ phenotype in gastric cancers may be independently predictive of lower lymph node metastasis and contribute to improved survival through tumor downstaging.
The MSI+ phenotype originates from the genetic or epigenetic inactivation of various members of the DNA mismatch repair gene family (31, 32). Recent studies have shown that the MSI+ phenotype of sporadic gastric cancers is mainly due to the inactivation of hMLH1 (33, 34). In this study, immunohistochemical staining revealed that 24 of 31 MSI+ cancers showed markedly diminished hMLH1 and 4 cases did not express hMSH2 protein, which is similar to results reported by other studies (29, 33, 34). In addition, loss of hMLH1 expression was also found in 4 of 235 MSI− gastric cancers. It is possible that MSI− cancers with diminished hMLH1 expression found in this study have been cancers with low frequency of MSI (35) or that hMLH1 expression loss has been over- or underestimated because of the limitation of tissue array methods.
The differences in MSI+ gastric cancers were restricted not only to the tumor phenotype but also to their genotype. MSI+ gastric cancers were reported to have fewer mutations of p53 and more frequent mutations of the TGFβRII gene than MSI− gastric cancers (28, 29). In this study, frameshift mutation analysis revealed that MSI+ gastric cancers displayed a higher frequency of target gene mutations of TGFβRII, BAX, hMSH3, and E2F4 genes. Of these different genotypes, mutations of the TGFβRII gene were mostly associated with patient survival in advanced carcinoma. TGFβ1 is a potent growth inhibitor, with tumor-suppressing activity. Recently, numerous experiments supported that TGFβ1 not only has transforming potential but can also drive malignant progression, invasion, and metastasis, both in vitro and in vivo (36). It therefore appears that complete abrogation of TGFβ signaling from mutations of the TGFβRII gene, although leading to loss of growth control and early tumor onset, paradoxically has a protective effect on tumor progression.
Gastric cancers have been found to contain higher levels of MUC1 mucin expression than normal gastric mucosa, and the expression of MUC2 mucin was observed frequently in intestinal type gastric cancer (37, 38). As MSI+ gastric cancers were significantly associated with intestinal subtype, it is expected that MSI+ gastric cancers showed higher expression of MUC2 mucin than MSI− cancers. Recently, CEA expression status in tumors was of prognostic significance in patients, and cytoplasmic expression was associated with poorly differentiated adenocarcinoma, more prominent serosal invasion, more frequent lymph node metastasis, and a more advanced stage (39). Although detailed mechanism was unknown, cytoplasmic expression of CEA were found less frequently in MSI+ gastric cancers.
In summary, we detected MSI+ phenotype in 9.5% of 327 gastric cancers. In our large and consecutive series, MSI+ gastric cancer had distinct clinical features, including old age, Borrmann's gross Type II, intestinal type, and lower prevalence of lymph node metastasis. The MSI+ phenotype in gastric cancers showed the survival advantage in a group with advanced gastric carcinoma. MSI+ phenotype is closely associated with hMLH1 or hMSH2 proteins.
References
Parkin DM, Pisani P, Ferlay J . Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer 1993; 54: 594–606.
Landis SH, Murray T, Bolden S, Wingo PA . Cancer statistics, 1998. CA Cancer J Clin 1998; 48: 6–29.
Huang JQ, Sridhar S, Chen Y, Hunt RH . Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998; 114: 1169–1179.
Ramon JM, Serra L, Cerdo C, Oromi J . Dietary factors and gastric cancer risk: a case-control study in Spain. Cancer 1993; 71: 1731–1735.
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998; 58: 5248–5257.
Aaltonen L, Peltomaki P, Leach FS, Sistonen P, Pylkkanan L, Mecklin JP, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260: 812–816.
Gryfe R, Kim H, Hsieh ETK, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000; 342: 69–77.
Kim H, Jen J, Vogelstein B, Hamilton SR . Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994; 145: 148–156.
Lynch HT, Smyrk TC, Watson P, Lanspa SJ, Lynch JF, Lynch PM, et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology 1993; 104: 1535–1549.
Zhou XP, Hoang JM, Li YJ, Seruca R, Carneiro F, Sobrinho-Simoes M, et al. Determination of the replication error phenotype in human tumors without the requirement for matching normal DNA by analysis of mononucleotide repeat microsatellites. Genes Chromosomes Cancer 1998; 21: 101–107.
Han HJ, Yanagisawa Q, Kato Y, Park JG, Nakamura Y . Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res 1993; 53: 5087–5089.
Strickler JG, Zheng J, Shu Q, Burgart LJ, Alberts SR, Shibata D . p53 mutations and microsatellite instability in sporadic gastric cancer: when guardians fail. Cancer Res 1994; 54: 4750–4755.
dos Santos NR, Seruca R, Constancia M, Seixas M, Sobrinho Simoes M . Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology 1996; 110: 38–44.
Wu MS, Lee CW, Shun CT, Wang HP, Lee WJ, Sheu JC, et al. Clinicopathological significance of altered loci of replication error and microsatellite instability-associated mutations in gastric cancer. Cancer Res 1998; 58: 1494–1497.
Kang GH, Yoon GS, Lee HK, Kwon YM, Ro JY . Clinicopathologic characteristics of replication error-positive gastric carcinoma. Mod Pathol 1999; 12: 15–20.
Kim HS, Woo DK, Bae SI, Kim YI, Kim WH . Microsatellite instability in the adenoma-carcinoma sequence of the stomach. Lab Invest 2000; 80: 57–64.
American Joint Committee on Cancer. AJCC cancer staging manual. 5th ed. Philadelphia: Lippincott-Raven; 1997.
Vasen HF, Mecklin JP, Khan PM, Lynch HT . The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991; 34: 424–425.
Dietmaier W, Wallinger S, Blocker T, Kullmann F, Fishel R, Ruschoff J . Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 1997; 57: 4749–4756.
Hoang JM, Cottu PH, Thuille B, Salmon RJ, Thomas G, Hamelin R . BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines. Cancer Res 1997; 57: 300–303.
Halling KC, Harper J, Moskaluk CA, Thibodeau SN, Petroni GR, Yustein AS, et al. Origin of microsatellite instability in gastric cancer. Am J Pathol 1999; 155: 205–211.
Samowitz WS, Slattery ML . Regional reproducibility of microsatellite instability in sporadic colorectal cancer. Genes Chromosomes Cancer 1999; 26: 106–114.
Kim WH, Lee HW, Park SH, Kim YI, Chi JG . Microsatellite instability in young age colorectal cancer. Pathol Int 1998; 48: 586–594.
Woo DK, Lee WA, Kim YI, Kim WH . Microsatellite instability and alteration of E2F-4 gene in adenosquamous and squamous cell carcinomas of the stomach. Pathol Int 2000; 50: 690–695.
Fearon ER, Vogelstein B . A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–767.
Loeb LA . Mutator phenotype may be required for multistage carcinogenesis. Cancer Res 1991; 51: 3075–3079.
Oliveira C, Seruca R, Seixas M, Sobrinho-Simoes M . The clinicopathological features of gastric carcinomas with microsatellite instability may be mediated by mutations of different “target genes”: a study of the TGFβRII, IGFIIR, and BAX genes. Am J Pathol 1998; 153: 1211–1219.
Yamamoto H, Perez-Piteira J, Yoshida T, Terada M, Itoh F, Imai K, et al. Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology 1999; 116: 1348–1357.
Wu MS, Lee CW, Shun CT, Wang HP, Lee WJ, Chang MC, et al. Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer 2000; 27: 403–411.
Noguchi Y, Yoshikawa T, Tsuburaya A, Motohashi H, Karpeh MS, Brennan MF . Is gastric carcinoma different between Japan and the United States? A comparison of patient survival among three institutions. Cancer 2000; 89: 2237–2246.
Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993; 75: 1027–1038.
Thibodeau SN, French AJ, Roche PC, Cunningham JM, Tester DJ, Lindor NM, et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res 1996; 56: 4836–4840.
Leung SY, Yuen ST, Chung LP, Chu KM, Chan ASY, Ho JCI . hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res 1999; 59: 159–164.
Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res 1999; 59: 1090–1095.
Pinto M, Oliveira C, Machado JC, Cirnes L, Tavares J, Carneiro F, et al. MSI-L gastric carcinomas share the hMLH1 methylation status of MSI-H carcinomas but not their clinicopathological profile. Lab Invest 2000; 80: 1915–1923.
Oft M, Heider KH, Beug H . TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol 1998; 8: 1243–1252.
Carrato C, Balague C, Bolos C, Gonzalez E, Gambus G, Planas J, et al. Differential apomucin expression in normal and neoplastic human gastrointestinal tissues. Gastroenterology 1994; 107: 160–172.
Baldus SE, Zirbes TK, Engel S, Hanisch FG, Mönig SP, Lorenzen J, et al. Correlation of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis of gastric carcinomas. Int J Cancer 1998; 79: 133–138.
Kim DY, Kim HR, Shim JH, Park CS, Kim SK, Kim YJ . Significance of serum and tissue carcinoembryonic antigen for the prognosis of gastric carcinoma patients. J Surg Oncol 2000; 74: 185–192.
Acknowledgements
This study was supported by the 21C Frontier Functional Human Genome Project of the Ministry of Science and Technology of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H., Choi, S., Lee, H. et al. Distinct Clinical Features and Outcomes of Gastric Cancers with Microsatellite Instability. Mod Pathol 15, 632–640 (2002). https://doi.org/10.1038/modpathol.3880578
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880578
Keywords
This article is cited by
-
Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer
Gastric Cancer (2019)
-
Molecular classification and precision therapy of cancer: immune checkpoint inhibitors
Frontiers of Medicine (2018)
-
MiR-27a rs895819 is involved in increased atrophic gastritis risk, improved gastric cancer prognosis and negative interaction with Helicobacter pylori
Scientific Reports (2017)
-
A protein and mRNA expression-based classification of gastric cancer
Modern Pathology (2016)
-
Epstein–Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer
Gastric Cancer (2016)