Abstract
Expression of adhesion molecule in low-grade B-cell mucosa-associated lymphoid tissue (MALT) lymphoma of the gastrointestinal tract has been reported in recent years, but these reports have primarily focused on low-grade gastrointestinal MALT lymphoma. In this study, we examined the lymphocytic homing receptor α4β7 integrin, L-selectin, and VLA-4 and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in low-grade lymphoma of the gastrointestinal tract and other organs such as the ocular adnexa and thyroid. We also observed changes in the expression pattern associated with high-grade transformation. Neoplastic cells in the gastrointestinal low-grade lymphoma and the low-grade component of high-grade MALT lymphoma were found to be α4β7 integrin+, L-selectin+, whereas the gastrointestinal high-grade component and diffuse large B-cell lymphoma were found to be α4β7 integrin−, L-selectin−. High endothelial venules in the gastric MALT lymphomas expressed MAdCAM-1. In the ocular adnexa low-grade MALT lymphoma, most cases were α4β7 integrin−, L-selectin+; and in the thyroid, most cases of both low- and high-grade MALT lymphoma were α4β7 integrin−, L-selectin−. These findings show that α4β7 integrin and L-selectin may play an important role in the lymphocyte homing of gastrointestinal low-grade MALT lymphoma and in the loss of α4β7 integrin expression throughout the course of high-grade progression.
Similar content being viewed by others
INTRODUCTION
Low-grade B-cell lymphomas of mucosa-associated lymphoid tissue (MALT) typically arise in extra-nodal organs that physiologically do not contain organized lymphoid tissues such as the gastrointestinal (G-I) tract, thyroid gland, orbits, salivary glands, respiratory tract, and genitourinary tract (1). MALT lymphomas tend to remain localized to the site of origin for long periods (2, 3). Lymph nodal involvement is rarely observed and only in cases of gastric low-grade MALT lymphoma invading the proper muscle layer of the stomach or deeper (4). However, MALT lymphomas sometimes disseminate to the MALT without lymph node involvement (5, 6, 7), as cutaneous lymphomas tend to spread to the skin without lymph nodal lesions. These observations imply that mechanisms controlling normal mucosal lymphocyte traffic may also be operational in MALT lymphomas.
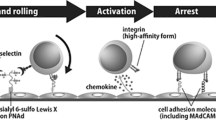
Lymphocyte homing into the selective lymphoid tissues is regulated by adhesion molecules expressed by the lymphocytes (homing receptors) and their endothelial ligands, vascular addressins (8, 9). It has been shown in mice that α4β7 integrin, which is expressed on mucosal lymphocytes, primarily mediates lymphocyte traffic to the intestinal mucosa through interactions with mucosal addressin cell adhesion molecule-1 (MAdCAM-1), which is expressed on high endothelial venules (HEVs) in the gut-associated lymphoid tissues (GALT; 10, 11). This has also been found to be the case in humans (12). The human α4β7 integrin is widely expressed by leukocyte subsets in the peripheral blood, organized lymphoid tissue, intestinal lamina propria, and lung parenchyma (12, 13, 14, 15, 16, 17). Its corresponding ligand, human MAdCAM-1, has recently been cloned and found to be expressed primarily in the small intestine and, to a lesser extent, in the colon and spleen (18, 19). Malignant lymphomas are expected to show the same expression pattern as that observed in corresponding counterparts of the nonneoplastic lymphoid tissues. In fact, α4β7 integrin is preferentially expressed in gastrointestinal low-grade MALT lymphomas (28). α4 integrin is also expressed on leukocytes as a heterodimeric integrin with the β1 integrin chain. α4β1 (VLA-4) is a ligand for VCAM-1 (20) and is involved in B-lymphocyte adhesion to germinal centers (21) but is not involved in lymphocyte homing to mucosal sites.
L-selectin regulates lymphocyte homing to the peripheral lymph node through interactions with the peripheral lymph node vascular addressin (PNAd) expressed on HEVs (8, 22). PNAds include GlyCAM-1 (23) and CD34 (24). Furthermore, L-selectin has been shown to bind to sialyl-Lewisx (25), which is also expressed on human HEV (26), and to a mucinlike domain of MAdCAM-1 (27). Therefore, it is possible that L-selectin is also involved in mucosal lymphocyte trafficking. L-selectin is expressed on germinal-center B cells and is consistently expressed on mantle zone B-cells (29). Although extrafollicular B cells and plasma cells in tonsils and lymph nodes are L-selectin positive, those within the lamina propria of gastrointestinal tract show a variable expression of L-selectin (29). As for malignant lymphomas, it has been reported that 50 to 80% of low-grade gastric lymphomas express L-selectin (29, 30).
Recent studies have revealed expression of α4β7 integrin and L-selectin in G-I MALT lymphomas (28, 29, 30), but there are few data about the homing receptors in MALT lymphomas of other organs. In the present study, we examined the expression patterns of α4β7 integrin, L-selectin, and VLA-4 in MALT lymphomas of the G-I tract, thyroid, and ocular adnexa. We also identified changes in their expression pattern associated with the high-grade transformation of low-grade MALT lymphoma.
MATERIALS AND METHODS
Tissue Samples
We examined 41 cases of low-grade MALT lymphoma (19 gastric, 3 intestinal, 10 ocular adnexal, and 9 thyroid), 9 cases of high-grade MALT lymphoma (5 gastric and 4 thyroid), and 19 cases of diffuse large B-cell lymphoma (DLBL; 8 gastric, 6 intestinal, 4 ocular adnexal, and 1 thyroid) selected from the files of the Second Department of Pathology, Okayama University Medical School. Low-grade MALT lymphomas were diagnosed according to the criteria described by Isaacson (1; Fig. 1A–B). We defined high-grade MALT lymphomas as combined lymphomatous foci of high-grade as well as low-grade components (Fig. 2A and D). Fresh tissue samples were obtained from the surgically resected materials, snap-frozen in liquid nitrogen, and stored at −80° C until used. For histopathological diagnosis, the tissue specimens were fixed in buffered formalin and paraffin embedded.
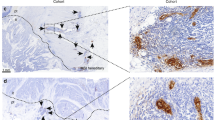
Immunohistochemical staining of α4β7 integrin and L-selectin in the high-grade gastric MALT lymphoma. A–C, low-grade components; D–F, high-grade components. A and D, histological pictures, 270 ×; B and E, α4β7 integrin, 160 ×; C and F, L-selectin, 160 ×. The high-grade components of high-grade MALT lymphoma are negative for either α4β7 integrin or L-selectin (E and F), whereas intermingled low-grade components and some intermingled nonneoplastic lymphocytes (upper right field) show positive immunoreactivity.
Immunohistochemistry
Immunohistochemical staining was performed on acetone-fixed cryostat sections for α4β7 integrin, L-selectin, and VLA-4 or on formalin-fixed, paraffin-embedded sections for MAdCAM-1 by the indirect immunoperoxidase method using dextran polymer–conjugated secondary antibody labeled with peroxidase (EnVision+, DAKO Japan, Kyoto, Japan). In brief, 4–6-μm-thick sections were blocked with 10% goat serum or 10% skim milk and then incubated with the primary antibodies listed in Table 1 for 90 minutes at room temperature. The specimens were then treated with the EnVision+ immunostaining system according to the manufacturer's instructions. Diaminobenzidine was used for color development, and hematoxylin, for counterstaining.
For double immunofluorescence, acetone-fixed frozen sections were blocked with 10% skim milk for 15 minutes and then treated with anti-α4β7 integrin monoclonal antibody (mAb) for 90 minutes at room temperature. After washing with 0.05% Tween 20-containing phosphate buffered saline (Tween-PBS), the specimens were treated with 1:100-diluted rhodamine–labeled goat (Fab′) 2 anti-mouse IgG (Leinco Technologies Inc., Ballwin, MO) for 30 minutes at room temperature. After washing with Tween-PBS and blocking with 10% normal mouse serum, they were treated with fluorescence isothiocyanate–labeled anti-CD 20 or anti-CD3 mAbs (DAKO) for 30 minutes at room temperature. Haexist 1:500 was used for nuclear staining. Simultaneous detection of both α4β7 integrin and L-selectin was performed by the double immunofluorescence method, as described elsewhere (31).
Statistical Analysis
A statistical comparison of α4β7 integrin or L-selectin immunoreactivity was performed by the χ2 test using a statistical software package (Fisher's exact test).
RESULTS
Expression of α4β7 Integrin in MALT Lymphoma
The immunoreactivity of MALT lymphomas and DLBL for α4β7 integrin, L-selectin, and VLA-4 is summarized in Table 2. Most of the low-grade gastric MALT lymphoma and a low-grade component of high-grade gastric MALT lymphoma were positive for α4β7 integrin (Fig. 1C and 2B). In contrast, a high-grade component of high-grade MALT lymphoma and DLBL rarely expressed α4β7 integrin (Fig. 2E), and this difference was statistically significant. α4β7 integrin was also found to be expressed in all cases of intestinal low-grade MALT lymphoma and in a small proportion of ocular adnexal and thyroid low-grade lymphoma. Nonneoplastic plasma cells in the lamina propria were positive for α4β7 integrin but negative for L-selectin. Some T cells were also positive for α4β7 integrin. MALT lymphoma cells frequently show plasmacytic differentiation and are sometimes intermingled with many reactive T cells; it is therefore critically important to determine whether α4β7 integrin-positive cells are centrocyte-like cells of MALT lymphoma, nonneoplastic plasma cells, or intermingled T cells. For this purpose, gastric low-grade MALT lymphoma tissues were immunostained by the double immunofluorescence method using fluorescence isothiocyanate–labeled anti-CD3 or CD20 mAb and anti-α4β7 integrin mAb combined with rhodamine-labeled anti-mouse IgG antibody. Figure 3A–B clearly shows that most α4β7 integrin–positive cells were concomitantly positive for CD20 and negative for CD3; they were thus easily discriminated from intermingled small-sized T cells.
Double immunofluorescence for α4β7 integrin and CD20, CD3, or L-selectin in low-grade gastric MALT lymphoma. (a), α4β7 (rhodamine), and CD20 (fluorescence isothiocyanate), 400 ×. (b), α4β7 (rhodamine) and CD3 (fluorescence isothiocyanate), 400 ×. (c), α4β7 (fluorescence isothiocyanate) and L-selectin (rhodamine), 600 ×. Double-positive cells are stained orange.
Expression of L-Selectin in MALT Lymphomas
We used two kinds of mAbs to L-selectin, DREG-56 and FMC46; FMC46 showed better reactivity, and the stainability of the specimens was evaluated from the results of staining with FMC46 (Table 2). L-selectin was frequently expressed in low-grade MALT lymphoma of the G-I tract and ocular adnexa and in a low-grade component of high-grade gastric MALT lymphoma (Figs. 1D and 2C), in contrast, a high-grade component of gastric high-grade MALT lymphoma tended to lose L-selectin expression (Fig. 2F). Low-grade MALT lymphomas of the thyroid infrequently expressed L-selectin, with L-selectin being detected in half of DLBL cases of the stomach and ocular adnexa but in none of DLBL cases of the gut and thyroid.
Expression of VLA-4 in MALT Lymphomas
VLA4 was detected in only a small proportion of the cases examined, but especially in cases of low-grade MALT lymphoma (Table 2). In gastric low-grade MALT lymphomas, three of four VLA-4+ cases were also positive for both α4β7 integrin and L-selectin.
Coexpression of α4β7 Integrin and L-Selectin
In the low-grade MALT lymphomas of the G-I tract, all 22 cases examined expressed α4β7 integrin, and 18 cases also expressed L-selectin. In the ocular adnexa and thyroid, approximately half of the cases of low-grade MALT lymphoma expressed either α4β7 integrin or L-selectin. In the cases expressing both α4β7 integrin and L-selectin, both adhesion molecules were primarily coexpressed at individual cellular levels, as evidenced by the double immunostaining (Fig. 3C), and only a few α4β7 integrin -positive cells were negative for L-selectin 2.
Expression of MAdCAM-1
MAdCAM-1 was consistently expressed on HEVs in the gastric mucosa of chronic gastritis and also in the lymphomatous lesions of gastric MALT lymphoma but not on HEVs in gastric DLBL (Fig. 4). HEVs in low-grade MALT lymphoma tissues of the ocular adnexa were negative for MAdCAM-1. In the thyroid, most lesions of chronic thyroiditis and about a half of low-grade MALT lymphoma lesions expressed MAdCAM-1 on the HEVs (data not shown).
DISCUSSION
In this study, we observed preferential expression of α4β7 integrin and L-selectin on lymphoma cells of low-grade MALT lymphoma and low-grade components of high-grade MALT lymphoma of the G-I tract. Plasma cells in the lamina propria of the human stomach and small intestine strongly expressed α4β7 integrin but were negative for L-selectin, as described elsewhere (30). Dogan et al. (30) have reported that the majority of marginal-zone B cells of the human GALT express low levels of α4β7 integrin and high levels of L-selectin. MALT lymphoma cells are derived from marginal-zone B cells of the memory phenotype. Therefore, it is reasonable that low-grade MALT lymphoma cells of the G-I tracts consistently expressed variable levels of α4β7 integrin and L-selectin. HEVs in the nonlymphomatous G-I mucosa and gastric MALT lymphoma tissues were positive for MAdCAM-1 as reported (30; Fig. 4). The interaction between the α4β7 integrin expressed by lymphocytes and MAdCAM-1 expressed by HEVs mediates lymphocyte homing to GALTs. This physiological homing mechanism may also function in MALT lymphomas and may explain the biological characteristic of MALT lymphomas in which they tend to remain localized to the site of origin for long periods.
Our results showing that low-grade MALT lymphoma cells of the G-I tract consistently express α4β7 integrin are comparable with those reported previously by Drillenburg et al. (28). High-grade components of gastric MALT lymphoma and DLBL cells were found to be consistently negative for α4β7 integrin. Thus, G-I MALT lymphoma cells may lose their expression of α4β7 integrin after progression to a high-grade malignancy. L-selectin was also found to be less frequently expressed in high-grade than in low-grade MALT lymphoma cells.
In contrast to the present data, Doganet al.,30) have reported that low-grade MALT lymphoma cells in the stomach are α4β7 integrin−, L-selectin+, whereas the intestinal secondary lymphoma cells are α4β7 integrin+, L-selectin−. They claimed that α4β7 integrin positivity in gastric low-grade MALT lymphoma may be overestimated by the presence of α4β7 integrin+ plasma cells in the lamina propria and that α4β7 integrin+ reactive T cells intermingling with lymphoma cells. In fact, some cases of gastric low-grade MALT lymphoma have shown substantial numbers of reactive T cells. In our series, however, lymphomatous lesions with only a few intermingled T cells also showed α4β7 integrin positivity. Even in the T cell–rich area, double immunofluorescent staining of CD3/α4β7 integrin and CD20/α4β7 integrin showed that CD20+ B cells were positive for α4β7 integrin.
In the ocular adnexa and thyroid, α4β7 integrin was less frequently expressed, even in low-grade MALT lymphomas. This evidence suggests that another homing system may operate in these organs, although MAdCAM-1 is frequently expressed in HEVs of the thyroid. VLA-4 was expressed only in a small proportion of low-grade MALT lymphomas and in none of the DLBL. Therefore, VLA-4 may not play a major role in the growth of lymphoma in the mucosal site.
In conclusion, the present study shows that α4β7 integrin and L-selectin may be involved in the mucosal homing of low-grade MALT lymphoma cells in the G-I tract and that the expression of α4β7 integrin is lost in the course of high-grade progression.
References
Isaacson PG, Norton AJ . Mucosa-associated lymphoid tissue (MALT) and the MALT lymphoma concept. In: Isaacson PG, Norton AJ, editors. Extranodal lymphomas. Edinburgh, United Kingdom: Churchill Livingstone; 1994. p. 5–114.
Isaacson PG . Gastrointestinal lymphoma. Hum Pathol 1994; 25: 1020–1029.
Krol AD, Hermans J, Kramer MH, et al. Gastric lymphomas compared with lymph node lymphomas in a population-based registry differ in stage distribution and dissemination pattern but not in patient survival. Cancer 1997; 79: 390–397.
Yoshino T, Akagi T . Gastric low-grade mucosa-associated lymphoid tissue lymphomas: their histogenesis and high-grade transformation. Pathol Int 1998; 48: 323–331.
Du MQ, Xu CF, Diss TC, Peng HZ, Wotherspoon AC, Isaacson PG, Pan LX . Intestinal dissemination of gastric mucosa-associated lymphoid tissue lymphoma. Blood 1996; 88: 4445–4451.
Mattia AR, Ferry JA, Harris NL . Breast lymphoma: a B-cell spectrum including the low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Am J Surg Pathol 1993; 17: 574–587.
Di Loreto C, Mariuzzi L, De Grassi A, Beltrami CA . B cell lymphoma of the thymus and salivary gland. J Clin Pathol 1996; 49: 595–597.
Picker LJ, Butcher EC . Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol 1992; 10: 561–591.
Butcher EC, Picker LJ . Lymphocyte homing and homeostasis. Science 1996; 272: 60–66.
Holzmann B, McIntyre BW, Weissman IL . Indentification of a murine Peyer's patch–specific lymphocyte homing receptor as an integrin molecule with an α chain homologous to human VLA-4α. Cell 1989; 56: 37–46.
Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993; 74: 185–195.
Erle DJ, Briskin MJ, Butcher EC, Garcia Pardo A, Lazarovits AI, Tidswell M . Expression and function of the MAdCAM-1 receptor, integrin α4β7, on human leukocytes. J Immunol 1994; 153: 517–528.
Schweighoffer T, Tanaka Y, Tidswell M, Erle DJ, Horgan KJ, Luce GE, et al. Selective expression of integrin α4β7 on a subset of human CD4+ memory T cells with hallmarks of gut-trophism. J Immunol 1993; 151: 717–729.
Postigo AA, Sanchez-Mateos P, Lazarovits AI, Sanchez-Madrid F, de Landazuri MO . α4β7-integrin mediate B cell binding to fibronectin and vascular cell adhesion molecule 1. Expression and function of α4 integrins on human B lymphocytes. J Immunol 1993; 151: 2471–2483.
Tiisala S, Paavonen T, Renkonen R . αEβ7 and α4β7 integrins associated with intraepithelial and mucosal homing, are expressed on macrophages. Eur J Immunol 1995; 25: 411–417.
Farstad IN, Halstensen TS, Lazarovits AI, Norstein J, Fausa O, Brandtzaeg P . Human intestinal B-cell blasts and plasma cells express the mucosal homing rectptor integrin α4β7. Scand J Immunol 1995; 42: 662–672.
Farstad IN, Halstensen TS, Kvale D, Fausa O, Brandtzaeg P . Topographic distribution of homing receptors of B and T cells in human gut-associated lymphoid tissue: relation of L-selectin and integrin α4β7 to naive and memory phenotypes. Am J Pathol 1997; 150: 187–199.
Shyjan A, Bertagnolli M, Kenney C, Briskin M . Human mucosal addressin cell adhesion molecule-1 (MAdCAM-1) demonstrates structural and functional similarities to the α4β7-integrin binding domain of murine MAdCAM-1, but extreme divergence of mucin-like sequences. J Immunol 1996; 156: 2851–2857.
Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997; 151: 97–110.
Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 1990; 60: 577–584.
Freedman AS, Munro JM, Rice GE, Bevilacqua MP, Morimoto C, McIntyre BW, et al. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science 1990; 249: 1030–1033.
Berg EL, Robinson MK, Warnock RA, Butcher EC . The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol 1991; 114: 343–349.
Lasky LA, Singer MS, Dowbenko D, Imai Y, Henzel WJ, Grimley C, et al. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell 1992; 69: 927–938.
Baumheter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, et al. Binding of L-selectin to the vascular sialomucin CD34. Science 1993; 262: 436–438.
Foxall C, Watson SR, Dowbenko D, Fennie C, Lasky LA, Kiso M, et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol 1992; 117: 895–902.
Munro JM, Lo SK, Corless C, Robertson MJ, Lee NC, Barnhill RL, et al. Expression of sialyl-Lewisx, an E-selectin ligand, in inflammation, immune responses, and lymphoid tissues. Am J Pathol 1992; 141: 1397–1408.
Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC . L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature 1993; 366: 695–698.
Drillenburg P, van der Voort R, Koopman G, Dragosics B, van Krieken JH, Kluin P, et al. Preferential expression of the mucosal homing receptor integrin α4β7 in gastrointestinal non-Hodgkin's lymphomas. Am J Pathol 1997; 150: 919–927.
Möller P, Eichelmann A, Mechtersheimer G, Koretz K . Expression of β1-integrins, H-CAM (CD44) and LECAM-1 in primary gastrointestinal B-cell lymphomas as compared to the adhesion receptor profile of the gut-associated lymphoid system, tonsil and peripheral lymph node. Int J Cancer 1991; 49: 846–855.
Dogan A, Du M, Koulis A, Briskin MJ, Isaacson PG . Expression of lymphocyte homing receptors and vascular addressins in low-grade gastric B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol 1997; 151: 1361–1369.
Teramoto N, Szekely L, Pokrovskaja K, Hu LF, Yoshino T, Akagi T, et al. Simultaneous detection of two independent antigens by double staining with two mouse monoclonal antibodies. J Virol Methods 1998; 73: 89–97.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (Grant-in-Aid for Exploratory Research 09877037).
We thank Dr. M.J. Briskin of LeukoSite Inc. for the generous gift of monoclonal antibody MAdCAM-1. We are also thankful to the Okayama University Medical School Hospital and the Aichi Cancer Center for providing the cases materials. We also thank Ms. Motochika for her help with the photography and Ms. Okabe for her help with the immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, YX., Yoshino, T., Ohara, N. et al. Loss of Expression of α4β7 Integrin and L-selectin Is Associated with High-Grade Progression of Low-Grade MALT Lymphoma. Mod Pathol 14, 798–805 (2001). https://doi.org/10.1038/modpathol.3880393
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880393
Keywords
This article is cited by
-
Mucosal Addressin Cell Adhesion Molecule-1 of Rhesus Macaques: Molecular Cloning, Expression, and Alteration After Viral Infection
Digestive Diseases and Sciences (2014)
-
Multiple mucosa-associated lymphoid tissue organs involving marginal zone B cell lymphoma: organ-specific relationships and the prognostic factors. Consortium for improving survival of lymphoma study
International Journal of Hematology (2010)
-
Duodenal and nodal follicular lymphomas are distinct: the former lacks activation-induced cytidine deaminase and follicular dendritic cells despite ongoing somatic hypermutations
Modern Pathology (2009)
-
Chlamydial infection: the link with ocular adnexal lymphomas
Nature Reviews Clinical Oncology (2009)
-
Deviated VH4 immunoglobulin gene usage is found among thyroid mucosa-associated lymphoid tissue lymphomas, similar to the usage at other sites, but is not found in thyroid diffuse large B-cell lymphomas
Modern Pathology (2006)