Abstract
Little is known about the association of angiomyolipoma and adult renal-cell neoplasia. We studied the clinicopathologic features of 36 patients with concurrent angiomyolipoma and renal-cell neoplasia from the consultation and surgical pathology files of nine institutions. HMB-45 immunoreactivity was analyzed in both neoplasms. Twenty-five sporadic cases of patients with angiomyolipoma and renal-cell neoplasia and 11 cases of patients with tuberous sclerosis, as defined by Gomez' criteria, had mean ages of 59 and 53 years, respectively, and female–male ratios of 2:1 and 5:1, respectively. The mean size of the angiomyolipomas was 1 cm in the sporadic cases and 3 cm in those patients with tuberous sclerosis (medians: 0.5 and 3 cm, respectively, P =.002). The mean sizes of the renal-cell neoplasms were 5 cm in sporadic cases and 6 cm in patients with tuberous sclerosis (medians: 4 and 5 cm, respectively; P =.88). In both clinical settings, angiomyolipoma was more commonly the incidental tumor. Clear-cell (conventional) renal-cell carcinoma was the most common renal-cell neoplasm in both groups of patients, accounting for approximately two thirds of the tumors. In patients with tuberous sclerosis, 27% of renal-cell neoplasms were oncocytomas, compared with 8% in sporadic cases (P =.15). Papillary neoplasia, chromophobe, and collecting-duct renal-cell carcinoma were found only in sporadic cases. All of the 22 renal-cell neoplasms studied were negative for HMB-45, whereas all 25 angiomyolipomas studied were positive.
Similar content being viewed by others
INTRODUCTION
The occurrence of angiomyolipoma in association with a renal-cell neoplasm is uncommon, with only approximately 50 cases reported (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37). Although angiomyolipomas are extremely common in patients with the tuberous sclerosis complex (38), it is unclear whether patients with tuberous sclerosis have more renal-cell neoplasms than the general population.
Our understanding of the biology and clinical behavior of angiomyolipoma and renal-cell neoplasia has recently been augmented by several important developments. There is strong evidence that angiomyolipomas, for a long time considered to be hamartomatous, are actually neoplasms (39, 40). The morphologic spectrum of angiomyolipoma has been recently expanded to include an epithelioid variant, which is capable of a more aggressive behavior than the classic form (41, 42, 43). Further, the recent description of the perivascular epithelioid cell family of tumors (44), to which angiomyolipoma belongs, has expanded our knowledge of entities that would fall into the differential diagnosis of angiomyolipoma and renal-cell neoplasms. Similarly, our knowledge of tuberous sclerosis has been strengthened by the definition of a constellation of phenotypic diagnostic criteria and the discovery of the genes involved in its pathogenesis (45, 46, 47). Last, our understanding of the biologic potential of renal-cell neoplasms has been recently expanded with the adoption of a widely accepted classification (48) that is based on distinct histologic features that correlate closely with consistent genetic abnormalities.
We undertook a detailed clinicopathologic study to ascertain the frequency of the concurrence of angiomyolipomas and renal-cell neoplasms in the same patient, whether any particular histologic type of renal-cell neoplasm is more frequently associated with angiomyolipomas, and whether the clinical presentation or behavior of either neoplasm is different compared with when they occur in isolation. Furthermore, we studied whether there was any difference in the presentation or behavior between patients with and without tuberous sclerosis.
MATERIALS AND METHODS
Surgical pathology records of nine institutions and the consultation files of the authors were searched for the diagnosis of renal angiomyolipoma. Patients that had coexistent, precedent, or subsequent renal-cell neoplasia were included in the study.
Clinical information was obtained from the patients' medical records and treating physicians and included age at presentation, gender, any personal or family history of tuberous sclerosis, and follow-up information. A patient was considered to have tuberous sclerosis if there was a clinical diagnosis of the disease or, according to Gomez' criteria (47), if there were multiple angiomyolipomas. Hematoxylin and eosin–stained sections of each neoplasm were reviewed. Renal epithelial neoplasms were classified according to the Rochester classification (48). Other pathologic features noted included size of the tumor and multifocality. Fuhrman nuclear grade and 1997 UICC/AJCC TNM pathologic stage were determined for malignant renal-cell neoplasms. Size, histologic type, and multifocality of angiomyolipoma were recorded. Because many of the cases were seen in consultation and all of the cases were retrieved retrospectively from nine institutions, gross sampling of tumors was not performed in any predictable, standardized manner.
At least one block from each neoplasm (available for 22 renal-cell neoplasms and 25 angiomyolipomas) was retrieved, and sections were submitted for immunohistochemical staining for monoclonal HMB-45 (DAKO, Carpenteria, CA; dilution, 1:40).
Statistical analysis was done with SPSS software, Version 7.0, for Windows 98. Fisher's Exact test was used to analyze frequency data, whereas the Student's t test was used for numerical data. P values of less than.05 were considered significant.
RESULTS
A total of 36 cases of coexistent renal-cell neoplasia and angiomyolipoma were identified from nine institutions and/or consultation files of the authors. Eighteen cases from five institutions were identified from a review of approximately 2160 nephrectomies performed for a renal mass, suggesting an incidence of slightly less than 1% (data were unavailable for the remaining four institutions). Clinical features are summarized in Table 1. In one case, the renal-cell neoplasm preceded a contralateral angiomyolipoma by 11 months, whereas in 35 cases, both neoplasms were diagnosed simultaneously. Additionally, one of these patients had a partial nephrectomy 6 years later for a contralateral angiomyolipoma. In 19 cases, both neoplasms occurred in the left kidney, in 10 they occurred in the right kidney, and in 4, the side was not specified. One patient had a renal-cell neoplasm in the left side and bilateral angiomyolipomas, and two patients had bilateral renal-cell neoplasms and an angiomyolipoma in the left kidney. One of the patients with bilateral renal-cell neoplasms had autosomal-dominant polycystic kidney disease.
Eleven patients (31%) had tuberous sclerosis. Of these, only six had a clinical diagnosis of tuberous sclerosis; in the remaining five, the diagnosis was made based on the presence of multiple angiomyolipomas (47). The mean ages for the sporadic and tuberous sclerosis-related cases, respectively, were 59 and 53 years (P =.365), and the female-to-male ratios were 2:1 and 5:1. Twenty-eight cases were managed with total nephrectomy (Fig. 1), seven with partial nephrectomy, and one with a radical and a contralateral partial nephrectomy. In both sporadic and tuberous sclerosis–associated patients, angiomyolipoma was more commonly the incidentally found tumor. This was also true within the category of tuberous sclerosis–associated patients (those with clinical history of tuberous sclerosis versus those assigned as tuberous sclerosis based on Gomez' criteria of multifocality).
For angiomyolipomas occurring sporadically, the mean and median sizes were 1.0 and 0.5 cm, respectively, compared with 3.3 and 2.7 cm for those occurring in association with tuberous sclerosis (P =.002). Within the tuberous sclerosis category, the mean size of tumors in patients with clinical history of tuberous sclerosis was 4.1 cm (range, 0.2 to 9.0 cm), whereas the mean size of tumors assigned as tuberous sclerosis based on multifocality (Gomez' criteria) was 2.8 cm (range, 0.5 to 5.5 cm). The mean and median sizes of renal-cell neoplasms in sporadic cases were 5.7 and 3.7 cm, respectively, and in tuberous sclerosis–associated cases, were 5.6 and 5.0 cm (P =.769). In patients with tuberous sclerosis, no renal-cell neoplasm was multifocal, whereas all 11 patients had multifocal angiomyolipomas. In contrast, in the sporadic setting, 7 of the 23 patients had multifocal renal-cell neoplasia, and none, by definition, had multifocal angiomyolipomas. In 11 malignant renal-cell neoplasms, the Fuhrman nuclear grade was 2; in 16 it was 3; and in 2 it was 4. Fifteen malignant neoplasms were stage pT1, three were stage pT2, five were pT3a, and four were pT3b, with one of them having metastases to multiple regional lymph nodes. In two cases, the pathologic stage could not be determined.
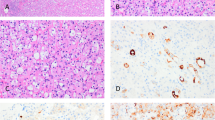
The distribution of cases by histologic subtype of the renal-cell neoplasm is presented in Table 2. Clear cell (conventional) renal-cell carcinoma was the most common histologic subtype in both sporadic and tuberous sclerosis-associated settings (60% and 64% of cases, respectively; Fig. 2). In patients with tuberous sclerosis, 27% of renal-cell neoplasms were oncocytomas, compared with 8% in sporadic cases (P =.15). When oncocytomas and chromophobe renal-cell carcinomas were grouped in a single category, 9 of 36 neoplasms (25%) belonged to this category (27% in patients with tuberous sclerosis, 24% in sporadic cases). Papillary neoplasia (adenoma or carcinoma), chromophobe renal-cell carcinoma, and collecting-duct renal-cell carcinoma were found only in sporadic cases. All renal-cell neoplasms studied by immunohistochemistry (n = 22) were negative for HMB-45, whereas all angiomyolipomas studied (n = 25) showed positive immunostaining in at least 5% of the cells.
Follow-up information was available in 14 patients (Table 3). Two patients died from metastatic renal-cell carcinoma and one from unknown causes; one developed pulmonary metastasis but has remained stable for 18 months since detection of the metastasis. The remaining 10 patients are alive with no evidence of disease.
DISCUSSION
Tuberous sclerosis is an autosomal-dominant disease, caused by abnormalities at two major loci: TSC1 at Chromosome 9q34 and TSC2 at Chromosome 16p13 (45, 46). Although many patients have the classical triad of mental retardation, seizures, and facial angiofibromas, it is well known that “formes fruste” of the disease exist (47). The constellation of lesions developed by these patients includes cerebral tubers, ungual fibromas, fibrous forehead plaques, and rhabdomyomas (38). The kidney is most commonly affected by the presence of angiomyolipomas, cysts, and less frequently carcinomas (49). In 1991, Gomez (47) published a list of clinical diagnostic criteria of the disease, including, within the “definitive” category, the presence of multiple angiomyolipomas.
Although most angiomyolipomas are composed of fat, smooth muscle, and abnormal blood vessels, the morphologic spectrum has been recently expanded with the recognition of the monotypic epithelioid variant (38, 41, 42, 43). These tumors have a striking resemblance to renal-cell carcinoma and often have high-grade histologic features, such as mitotic activity, pleomorphism, and necrosis. Epithelioid angiomyolipoma is malignant and has caused death in 35%–50% of reported cases (38, 41). Although specific histologic features have been described, immunohistochemistry also is helpful for its diagnosis, including negative reactivity with anticytokeratin antibodies, positive reaction with antibodies to actin, and consistent expression of melanogenic markers, particularly HMB-45, tyrosinase, and microphthalmia transcription factor (50). Other unusual forms of angiomyolipoma include the lipomatous and leiomyomatous variants, which resemble lipoma or well-differentiated liposarcoma, and leiomyoma or leiomyosarcoma, respectively (51, 52, 53).
Whether the incidence of renal-cell carcinoma is increased in patients with tuberous sclerosis is still a matter of debate. In the Eker rat, a germline mutation in the TSC2 gene is associated with autosomal-dominant hereditary renal carcinoma (54). In 1998, Tello et al. (55) conducted a meta-analysis of the relationship between tuberous sclerosis and renal-cell carcinoma and concluded that tuberous sclerosis patients had no increased risk for the development of renal-cell carcinoma. Further, Pea et al. (43) analyzed five cases previously reported as renal-cell carcinoma in tuberous sclerosis and found three of them to be epithelioid angiomyolipomas, suggesting that the incidence of tuberous sclerosis–associated renal-cell carcinoma is lower than some have suggested (4). We have also seen in consultation cases originally diagnosed as renal-cell carcinomas that turned out to be epithelioid angiomyolipomas. Nevertheless, it has been reported in the literature that isolated renal-cell carcinoma in tuberous sclerosis occurs at a younger age, is more frequent in females, and has a relatively favorable clinical outcome compared with renal-cell carcinoma in the general population (4, 56).
The coexistence of angiomyolipoma and renal-cell neoplasia is uncommon, with slightly more than 50 reported cases in the world literature (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37). Clinicopathologic analysis of these case reports is difficult because most have been published in clinical journals and lack detailed pathologic descriptions and photomicrographs from which histologic type could be confidently discerned. Second, most reports preceded the current classification of renal-cell neoplasia, and histologic typing of the tumors was not performed in most. Third, the studies lack uniform criteria regarding the diagnosis of tuberous sclerosis. Finally, most were published before epithelioid angiomyolipoma was recognized. Despite these obstacles, some conclusions can be drawn from the literature. Of 52 reported cases, 34 (63%) were identifiable as clear-cell renal-cell carcinoma; 2 (4%) were papillary (one of them with a coexistent clear-cell renal-cell carcinoma); 2 (4%) were urothelial carcinomas; 6 (12%) were oncocytomas (one with a concurrent transitional cell carcinoma); and in 10 (19%), the tumor was not classified or could not be classified with the photomicrographs provided. One of the cases reported as a clear-cell renal-cell carcinoma was later reclassified by Pea et al. as an epithelioid angiomyolipoma (43). Twenty-nine cases (56%; 22 females, 7 males) were associated with tuberous sclerosis, whereas 23 (44%; 15 females, 8 males) were sporadic. The mean age for cases related to tuberous sclerosis was 32 ± 16 years, and for sporadic cases, 57 ± 11 years.
In the present study, the cases have been analyzed using uniform criteria both for the definition of tuberous sclerosis and for the types of renal-cell neoplasms. The only caveat in considering our data is the fact that all cases were retrospectively acquired. Because one of the features determining inclusion of a case in the tuberous sclerosis category was multifocality, it is conceivable that a case considered by us as sporadic by virtue of the presence of a single angiomyolipoma recorded at the time of the original reporting may in fact have represented a familial case, if extensive sampling of the kidney had been performed and multifocality demonstrated. We encountered a smaller proportion of tuberous sclerosis–related cases compared with that in previous reports (32% versus 44%). Further, unlike in previously reported cases, we did not find a significant difference in the age of presentation of coexistent angiomyolipomas and renal-cell neoplasms in patients with and without tuberous sclerosis. Coexistent neoplasms were found more frequently in women in keeping with a higher incidence of angiomyolipomas in women. This sex predilection was stronger among patients with tuberous sclerosis. We found no significant difference in the size of the renal-cell neoplasms between sporadic and tuberous sclerosis–related cases.
In sporadic cases, as well as in the setting of tuberous sclerosis, the most common renal-cell neoplasm is clear-cell renal-cell carcinoma. This observation parallels the fact that in the general population, clear-cell renal-cell carcinoma is the most common renal-cell carcinoma. However, it is interesting that in patients with tuberous sclerosis, there was a higher (although not statistically significant) frequency of oncocytomas than in the sporadic cases. Whether this trend would hold in a larger series of cases is unknown. Against this is the observation that all oncocytomas previously reported to be associated with an angiomyolipoma have presumably occurred in sporadic settings (3, 23, 26, 29, 34). There are also a few reports of oncocytomas not associated with angiomyolipoma arising in patients with tuberous sclerosis (57, 58, 59), but overall, a higher incidence of oncocytoma in tuberous sclerosis patients has not previously been reported. We also encountered one case of coexistent metanephric adenoma and angiomyolipoma in a patient with tuberous sclerosis. The absence of other subtypes of renal-cell neoplasia associated with angiomyolipoma in patients with tuberous sclerosis suggests that there is no significant association between these neoplasms and tuberous sclerosis. Thus, our data suggest that the only two renal-cell neoplasms in which the tuberous sclerosis genomic alteration could potentially play a contributing role in their pathogenesis are clear-cell renal-cell carcinoma and oncocytoma. This concept is further supported by the fact that all previously reported cases of coexistent angiomyolipomas and renal-cell neoplasia in patients with tuberous sclerosis have been of the clear-cell or unclassified type, except one case of papillary renal-cell carcinoma that coexisted in a kidney with an angiomyolipoma and a clear-cell renal-cell carcinoma (2). Notwithstanding, there have been other types of renal-cell neoplasia (not associated with angiomyolipoma) reported in the patients with tuberous sclerosis, including chromophobe renal-cell carcinoma (60).
Another intriguing observation in our series is the fact that nine of the 36 cases studied (25%) were oncocytomas or chromophobe renal-cell carcinomas. This significantly increased incidence was seen both in sporadic and tuberous sclerosis cases. Recently, in one of the coauthors' experience (MBA) with a series of 405 consecutive cases of renal-cell neoplasms from a single institution (61), the combined proportion of renal oncocytoma and chromophobe renal-cell carcinoma was 12.6%, compared with 25% in the present study (P =.04). The rationale to group these two kinds of tumors is the emerging evidence that both entities may be in fact related from the histogenetic standpoint. Both chromophobe renal-cell carcinomas and renal oncocytomas are derived from the intercalated cells of the collecting ducts (62, 63); share some cytogenetic abnormalities (64); have overlapping morphologic (65, 66), immunohistochemical (67), and ultrastructural (68) features; and occur in cases of renal oncocytosis (69). Similarly, in the previously reported cases in the literature of coexisting angiomyolipomas and renal-cell neoplasia, an unusually high incidence of oncocytomas associated with angiomyolipoma is also seen (12%). Whether this overrepresentation of this group of neoplasms in our series and/or in the collective literature is secondary to selection/reporting bias or, conversely, has true pathogenetic implications is unknown at this point.
Finally, our limited follow-up data precludes an accurate analysis of the prognosis of this association of neoplasms or a comparison with isolated renal-cell neoplasia, although it appears that clinical outcome, as expected, is mostly dependent on pathologic stage of the renal-cell carcinomas.
In summary, we have identified 36 cases of coexistent angiomyolipoma and renal-cell neoplasia from the surgical pathology files of nine institutions and consultation files of the authors, rendering the largest series to date of this unique association of neoplasms. Most of our cases occurred in patients without a history of tuberous sclerosis. Although the most common subtype in both sporadic and tuberous sclerosis-associated settings was clear-cell (conventional) renal-cell carcinoma, a slightly higher proportion of oncocytomas and chromophobe renal-cell carcinomas was seen in both settings, compared with their combined prevalence in a series of sporadic renal-cell carcinomas (61). This observation warrants further study regarding a possible shared mechanism in the pathogenesis of angiomyolipoma and renal oncocytoma, as well as chromophobe renal cell carcinoma.
References
Ahuja S, Loffler W, Wegener OH, Ernst H . Tuberous sclerosis with angiomyolipoma and metastasized hypernephroma. Urology 1986; 28: 413–419.
Barbour GL, Casali RE . Bilateral angiomyolipomas and renal cell carcinoma in polycystic kidney. Urology 1978; 12: 694–698.
Berlizot P, Peyret C, Beddouch A, Thiounn N, Flam T, Zerbib M, et al. [Association of angiomyolipoma and oncocytoma of the kidney. Apropos of two cases.] J Urol (Paris) 1993; 99: 47–50.
Bjornsson J, Short MP, Kwiatkowski DJ, Henske EP . Tuberous sclerosis-associated renal cell carcinoma. Clinical, pathological, and genetic features. Am J Pathol 1996; 149: 1201–1208.
Blute ML, Malek RS, Segura JW . Angiomyolipoma: clinical metamorphosis and concepts for management. J Urol 1988; 139: 20–24.
Canzonieri V, Volpe R, Gloghini A, Carbone A, Merlo A . Mixed renal tumor with carcinomatous and fibroleiomyomatous components, associated with angiomyolipoma in the same kidney. Pathol Res Pract 1993; 189: 951–956.
Conrad S, Wagner B, Hamper K, Henke RP . [Kidney cancer and angiomyolipoma in lymphangiomyomatosis.] Urologe A 1994; 33: 76–79.
Csanaky G, Szereday Z, Magyarlaki T, Mehes G, Herbert T, Buzogany I . Renal angiomyolipoma: report of three cases with regional lymph node involvement and/or with renal cell carcinoma. Tumori 1995; 81: 469–474.
Graves N, Barnes WF . Renal cell carcinoma and angiomyolipoma in tuberous sclerosis: case report. J Urol 1986; 135: 122–123.
Gutierrez OH, Burgener FA, Schwartz S . Coincident renal cell carcinoma and renal angiomyolipoma in tuberous sclerosis. AJR Am J Roentgenol 1979; 132: 848–850.
Hardman JA, McNicholas TA, Kirkham N, Fletcher MS . Recurrent renal angiomyolipoma associated with renal carcinoma in a patient with tuberous sclerosis. Br J Urol 1993; 72: 983–984.
Honey RJ, Honey RM . Tuberose sclerosis and bilateral renal carcinoma. Br J Urol 1977; 49: 441–446.
Huang JK, Ho DM, Wang JH, Chou YH, Chen MT, Chang SS . Coincidental angiomyolipoma and renal cell carcinoma—report of 1 case and review of literature. J Urol 1988; 140: 1516–1518.
Jochimsen PR, Braunstein PM, Najarian JS . Renal allotransplantation for bilateral renal tumors. JAMA 1969; 210: 1721–1724.
Kavaney PB, Fielding I . Angiomyolipoma and renal cell carcinoma in same kidney. Urology 1975; 6: 643–646.
Lynne CM, Carrion HM, Bakshandeh K, Nadji M, Russel E, Politano VA . Renal angiomyolipoma; polycystic kidney, and renal cell carcinoma in patient with tuberous sclerosis. Urology 1979; 14: 174–176.
Mai KT, Perkins DG, Robertson S, Thomas J, Morrash C, Collins JP . Composite renal cell carcinoma and angiomyolipoma: a study of the histogenetic relationship of the two lesions. Pathol Int 1999; 49: 1–8.
Malone MJ, Johnson PR, Jumper BM, Howard PJ, Hopkins TB, Libertino JA . Renal angiomyolipoma: 6 case reports and literature review. J Urol 1986; 135: 349–353.
Mazeman E, Wemeau L, Biserte J, Riquet D . Renal angiomyolipoma. A report of 11 cases. Eur Urol 1980; 6: 328–334.
Morita T, Hirota N, Tokue A . Association of renal cell carcinoma and angiomyolipoma in the same kidney. Urol Int 1988; 43: 297–298.
Nakamura Y, Murayama K, Katsumi T, Watanabe K, Kishitani M, Ohkawa M . Coincident renal cell carcinoma and renal angiomyolipoma in tuberous sclerosis: a case report. Acta Urol Jpn 1994; 40: 703–706.
Ohigashi T, Iigaya T, Hata M . Coincidental renal cell carcinoma and renal angiomyolipomas in tuberous sclerosis. Urol Int 1991; 47: 160–163.
Schneck FX, Banner BF, Bahnson RR . Multiple renal neoplasms: a case of 3 histologically dissimilar primary tumors. J Urol 1991; 145: 1251–1253.
Schujman E, Meiraz D, Liban E, Servadio C . Mixed renomedullary tumor: renal cell carcinoma associated with angiomyolipoma. Urology 1981; 17: 375–376.
Silpananta P, Michel RP, Oliver JA . Simultaneous occurrence of angiomyolipoma and renal cell carcinoma. Clinical and pathologic (including ultrastructural) features. Urology 1984; 23: 200–204.
Siracusano S, Zanon M, D'Aloia G, Plaino F, Trombetta C, Bussani R . Rare association of renal angiomyolipoma and oncocytoma. Urology 1998; 51: 837–839.
Sugimoto M, Takamura S . Renal angiomyolipoma and renal cell carcinoma associated with tuberous sclerosis: a case report. Acta Urol Jpn 1997; 43: 33–35.
Takeyama M, Arima M, Sagawa S, Sonoda T . Preoperative diagnosis of coincident renal cell carcinoma and renal angiomyolipoma in nontuberous sclerosis. J Urol 1982; 128: 579–581.
Talic RF, el Faqih SR, al Rikabi AC, Ekman P . Rare association of unilateral renal oncocytoma and angiomyolipoma. Scand J Urol Nephrol 1997; 31: 91–93.
Taylor RS, Joseph DB, Kohaut EC, Wilson ER, Bueschen AJ . Renal angiomyolipoma associated with lymph node involvement and renal cell carcinoma in patients with tuberous sclerosis. J Urol 1989; 141: 930–932.
Tsuboniwa N, Meguro N, Nakamura Y, Maeda O, Saiki S, Kinouchi T, et al. Coexistence of renal cell carcinoma and renal angiomyolipoma developing in a kidney: a case report. Acta Urol Jpn 1997; 43: 131–135.
Ueda J, Kobayashi Y, Itoh H, Itatani H . Angiomyolipoma and renal cell carcinoma occurring in same kidney: CT evaluation. J Comput Assist Tomogr 1987; 11: 340–341.
Veiga FG, Varela JR, Chantada V, Martin MG . Coincidental renal angiomyolipoma and urothelial carcinoma of the renal pelvis in the same kidney diagnosed by CT. Br J Urol 1990; 66: 216–217.
Waters DJ, Holt SA, Andres DF . Unilateral simultaneous renal angiomyolipoma and oncocytoma. J Urol 1986; 135: 568–570.
Wei CH, Chen A, Tseng HH, Tu YC, Yen CY . Simultaneous renal cell carcinoma and angiomyolipoma in a kidney—a case report and literature review. Chung Hua I Hsueh Tsa Chih (Taipei) 1990; 45: 126–129.
Weinblatt ME, Kahn E, Kochen J . Renal cell carcinoma in patients with tuberous sclerosis. Pediatrics 1987; 80: 898–903.
Yamanaka N, Imai T, Fujisawa M, Kamidono S . Renal cell carcinoma and angiomyolipoma in tuberous sclerosis. Case report. Nippon Hinyokika Gakkai Zasshi 1990; 81: 304–307.
Eble JN . Angiomyolipoma of kidney. Semin Diagn Pathol 1998; 15: 21–40.
Green AJ, Sepp T, Yates JR . Clonality of tuberous sclerosis harmatomas shown by non-random X-chromosome inactivation. Hum Genet 1996; 97: 240–243.
Kattar MM, Grignon DJ, Eble JN, Hurley PM, Lewis PE, Sakr WA, et al. Chromosomal analysis of renal angiomyolipoma by comparative genomic hybridization: evidence for clonal origin. Hum Pathol 1999; 30: 295–299.
Eble JN, Amin MB, Young RH . Epithelioid angiomyolipoma of the kidney: a report of five cases with a prominent and diagnostically confusing epithelioid smooth muscle component. Am J Surg Pathol 1997; 21: 1123–1130.
Mai KT, Perkins DG, Collins JP . Epithelioid cell variant of renal angiomyolipoma. Histopathology 1996; 28: 277–280.
Pea M, Bonetti F, Martignoni G, Henske EP, Manfrin E, Colato C, et al. Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J Surg Pathol 1998; 22: 180–187.
Bonetti F, Pea M, Martignoni M, Zamboni G, Manfrin E, Colombari R, et al. The perivascular epithelioid cell and related lesions. Adv Anat Pathol 1994; 4: 343–358.
Povey S, Armour J, Farndon P, Haines JL, Knowles M, Olopade F, et al. Report and abstracts of the Third International Workshop on Chromosome 9. Cambridge, United Kingdom, 9–11 April, 1994. Ann Hum Genet 1994; 58: 177–250.
The European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993; 75: 1305–1315.
Gomez MR . Phenotypes of the tuberous sclerosis complex with a revision of diagnostic criteria. Ann N Y Acad Sci 1991; 615: 1–7.
Störkel S, Eble JN, Adlakha K, Amin MB, Blute ML, Bostwick DG, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997; 80: 987–989.
Bernstein J, Robbins TO . Renal involvement in tuberous sclerosis. Ann N Y Acad Sci 1991; 615: 36–49.
Zavala-Pompa A, Folpe AL, Jimenez RE, Lim SD, Cohen C, Eble JN, et al. Immunohistochemical study of microphthalmia transcription factor and tyrosinase in angiomyolipoma of the kidney, renal cell carcinoma, and renal and retroperitoneal sarcomas: comparative evaluation with traditional diagnostic markers. Am J Surg Pathol 2001; 25: 65–70.
Nonomura A, Minato H, Kurumaya H . Angiomyolipoma predominantly composed of smooth muscle cells: problems in histological diagnosis. Histopathology 1998; 33: 20–27.
Enzinger F, Weiss S . Soft tissue tumors, 3rd ed. St. Louis, MO: Mosby-Year Book, Inc.; 1995.
Bonsib SM . HMB-45 reactivity in renal leiomyomas and leiomyosarcomas. Mod Pathol 1996; 9: 664–669.
Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG . Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous scle-rosis 2 (TSC2) gene. Proc Natl Acad Sci U S A 1994; 91: 11413–11416.
Tello R, Blickman JG, Buonomo C, Herrin J . Meta analysis of the relationship between tuberous sclerosis complex and renal cell carcinoma. Eur J Radiol 1998; 27: 131–138.
Washecka R, Hanna M . Malignant renal tumors in tuberous sclerosis. Urology 1991; 37: 340–343.
Srinivas V, Herr HW, Hajdu EO . Partial nephrectomy for a renal oncocytoma associated with tuberous sclerosis. J Urol 1985; 133: 263–265.
Sugao H, Takiuchi H, Takatera H, Yokokawa K, Sakurai T, Kobayashi Y . [Renal oncocytoma associated with tuberous sclerosis: report of a case.] Hinyokika Kiyo 1987; 33: 1411–1415.
Green JA . Renal oncocytoma and tuberous sclerosis. A case report. S Afr Med J 1987; 71: 47–48.
Hidai H, Chiba T, Takagi Y, Taki A, Nagashima Y, Kuroko K . Bilateral chromophobe cell renal carcinoma in tuberous sclerosis complex. Int J Urol 1997; 4: 86–89.
Amin M, Tamboli P, Javidan J, Deshpande A, Farah R, Menon M, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms [abstract]. Mod Pathol 1998; 11: 75A.
Störkel S, Steart PV, Drenckhahn D, Thoenes W . The human chromophobe cell renal carcinoma: its probable relation to intercalated cells of the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol 1989; 56: 237–245.
Lyzak JS, Farhood A, Verani R . Intracytoplasmic lumens in renal oncocytoma and possible origin from intercalated cells of the collecting duct. J Urol Pathol 1994; 2: 135–151.
Dijkhuizen T, van den Berg E, Störkel S, de Vries B, van der Veen AY, Wilbrink M, et al. Renal oncocytoma with t(5;12;11), der(1)1;8) and add(19): “true” oncocytoma or chromophobe adenoma? Int J Cancer 1997; 73: 521–524.
Tickoo SK, Amin MB . Discriminant nuclear features of renal oncocytoma and chromophobe renal cell carcinoma. Analysis of their potential utility in the differential diagnosis. Am J Clin Pathol 1998; 110: 782–787.
Cochand-Priollet B, Molinie V, Bougaran J, Bouvier R, Dauge-Geffroy MC, Deslignieres S, et al. Renal chromophobe cell carcinoma and oncocytoma. A comparative morphologic, histochemical, and immunohistochemical study of 124 cases. Arch Pathol Lab Med 1997; 121: 1081–1086.
Beham A, Ratschek M, Zatloukal K, Schmid C, Denk H . Distribution of cytokeratins, vimentin and desmoplakins in normal renal tissue, renal cell carcinomas and oncocytoma as revealed by immunofluorescence microscopy. Virchows Arch A Pathol Anat Histopathol 1992; 421: 209–215.
Tickoo SK, Lee MW, Eble JN, Amin M, Christopherson T, Zarbo RJ, et al. Ultrastructural observations on mitochondria and microvesicles in renal oncocytoma, chromophobe renal-cell carcinoma, and eosinophilic variant of conventional (clear cell) renal-cell carcinoma. Am J Surg Pathol 2000; 24: 1247–1256.
Tickoo SK, Reuter VE, Amin MB, Srigley JR, Epstein JI, Min KW, et al. Renal oncocytosis: a morphologic study of fourteen cases. Am J Surg Pathol 1999; 23: 1094–1101.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 89th United States and Canadian Academy of Pathology Annual Meeting, New Orleans, LA, March 21–31, 2000.
Rights and permissions
About this article
Cite this article
Jimenez, R., Eble, J., Reuter, V. et al. Concurrent Angiomyolipoma and Renal Cell Neoplasia: A Study of 36 Cases. Mod Pathol 14, 157–163 (2001). https://doi.org/10.1038/modpathol.3880275
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880275
Keywords
This article is cited by
-
Renal collision tumours: three additional case reports
BMC Urology (2022)
-
Renal tumors in tuberous sclerosis complex
Pediatric Nephrology (2021)
-
Clinical and morphologic review of 60 hereditary renal tumors from 30 hereditary renal cell carcinoma syndrome patients: lessons from a contemporary single institution series
Medical Oncology (2019)
-
Renal disease in tuberous sclerosis complex: pathogenesis and therapy
Nature Reviews Nephrology (2018)
-
Hepatic angiomyolipoma: differential diagnosis from other liver tumors in a special reference to vascular imaging - importance of early drainage vein
Surgical Case Reports (2015)