Abstract
Synovial sarcoma in its classic biphasic form can be distinguished readily from other soft tissue lesions; however, monophasic and poorly differentiated forms are diagnostically more problematic. For this reason, we assessed the efficacy of immunostaining for SYT and SSX1 proteins, the gene products resulting from unique synovial sarcoma translocation, to distinguish synovial sarcoma from other soft tissue lesions. A total number of 146 cases were analyzed, including 47 synovial sarcoma cases (all of which were verified by FISH to have t(X; 18) translocation and SYT–SSX fusion gene) and 99 soft tissue tumors of various types. A polyclonal IgG antibody against SYT was used to stain formalin-fixed paraffin embedded tissues. Forty-one out of 47 (87%) synovial sarcoma displayed strong positive nuclear staining (ranging from 80 to 90% of the tumor cells) for SYT antibody. Nineteen of 99 (19%) non-synovial sarcoma cases showed variable nuclear and cytoplasmic staining with SYT, which ranged from 20 to 60% of tumor nuclei, and included malignant peripheral nerve sheath tumor (5/25), solitary fibrous tumor (2/14), Ewing sarcoma (2/6), low grade fibromyxoid tumor (2/4), extraskeletal mesenchymal chondrosarcoma (2/6), gastrointestinal tumor (4/17), epithelioid sarcoma (2/2). The remaining non-synovial sarcomas were negative. This is the first study demonstrating SYT protein expression in tissue sections of synovial sarcoma. This method could provide an easy, rapid and widely applicable means of assisting in the diagnosis of synovial sarcoma, particularly when material and/or resources are unavailable for PCR or FISH-based testing. However, as variable weak staining for SYT may be encountered in a small percentage of non-synovial sarcoma sarcomas, a positive interpretation should be made only when the staining is strong, nuclear and present in the majority of cells.
Similar content being viewed by others
Main
Synovial sarcoma is a distinct soft tissue neoplasm, which represents 7–10% of all human soft tissue sarcomas.1 In its classic, biphasic form, consisting of well-differentiated glands embedded within a spindled sarcomatous stroma, it is relatively easy to recognize. However, two-thirds of synovial sarcomas are of the monophasic fibrous type and consist predominantly or exclusively of a sarcomatous stroma without glands and, therefore, closely resemble other sarcomas, notably fibrosarcoma. In these situations, immunohistochemistry using a panel of antibodies for cytokeratin, EMA, CD99 and bcl-2 are commonly employed but are not fully specific. On the other hand, identification of the reciprocal chromosomal translocation t(x; 18)(p11.2; p11.2), which occurs in more than 90% of synovial sarcomas,2, 3, 4, 5, 6, 7 by FISH or RT-PCR is highly specific, but often not available in many laboratories. Therefore, development of an immunohistochemical stain highly specific for synovial sarcoma would be useful and practical.
It is now generally accepted that synovial sarcoma is derived from putative multipotent stem cells that are capable of differentiating into mesenchymal and/or epithelial structures.8 The reciprocal chromosomal translocation t(x; 18)(p11.2; p11.2) results in new fusion genes SYT–SSX1, SYT–SSX2 or SYT–SSX4 composed of the N-terminal part of the SYT gene on chromosome 18 with C-terminal part of either SSX1, SSX2, or SSX4 genes on chromosome X.8, 9, 10 The SYT–SSX fusion genes encode proteins comprised of the first 379 amino acids of SYT juxtaposed to the last 78 amino acids of either SSX1 or SSX2.8, 9, 10 Owing to an N-terminal domain encompassing amino acids 51–90 in SYT sequence and the presence of the highly conserved SSX-RD domain in the SSX sequence, both are responsible for their nuclear localization. The SYT, the SSX and the SYT–SSX fusion protein are all localized in the nucleus of synovial sarcoma cells.11, 12 Studies have indicated that the SYT proteins may function as a transcriptional activator, whereas the SSX proteins may act as transcriptional repressor. The fusion protein may act as a transcriptional regulator through interaction with other proteins to trigger synovial sarcoma development and proliferation.12, 13 As most translocations in various tumors show expression of partner genes involved with the translocation, it is reasonable to suspect common expression of SYT and SSX proteins in synovial sarcomas.
We have, therefore, assessed the sensitivity and specificity of immunostaining for SYT protein, one of the gene products resulting from the unique synovial sarcoma translocation, using a polyclonal antibody originally developed against the SYT protein in formalin-fixed, paraffin-embedded tissue sections of synovial sarcoma and other types of soft tissue tumors.
Materials and methods
Case Selection
To perform this study, we used two sets of materials: one set was the tissue microarray sections, including synovial sarcomas and various types of other soft tissue tumors; another set was conventional tissue sections of retrieved archived paraffin-embedded blocks of synovial sarcoma cases. We retrieved archival paraffin-embedded blocks of 12 synovial sarcoma cases from the Department of Pathology at the Loyola University Medical Center that were reported in the last 16 years (1990–2006). These 12 synovial sarcoma cases include nine cases of monophasic (MSS) phenotype, two cases of biphasic (BSS) phenotype and one case of poorly differentiated phenotype (Table 1). The tissue array slides with soft tissue tumors contained a total number of 134 cases and 16 different types of soft tissue tumors obtained from the Cleveland Clinic, Cleveland, OH (Table 2). This array included 35 synovial sarcoma, 25 malignant peripheral nerve sheath tumor, 17 GIST (gastrointestinal stromal tumors), 14 SFT (solitary fibrous tumors), six Ewing sarcoma/PNET (primitive neuroectodermal tumor), six alveolar rhabdomyosarcoma, six extraskeletal mesenchymal chondrosarcoma, four hemangiopericytoma, four myxoid liposarcoma, three desmoplastic small round cell tumor and two each of leiomyosarcoma, dedifferentiated liposarcoma, malignant melanoma, neuroblastoma and epithelioid sarcoma (Table 2). Frozen and formalin fixed cell blocks of an established synovial sarcoma cell line (BAC) containing SYT–SSX1 fusion gene was also included in the study.
Immunohistochemistry
Archival paraffin-embedded tissue blocks were cut into 4–6 μm sections and transferred to glass slides. After deparaffinization, antigen retrieval was carried out using 0.1 mol/l concentration of citrate buffer at pH 6.0, with microwaving for 30 min. The slides were stained on an automated Ventana NEXES immunostainer.
The polyclonal anti-SYT antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at 1:250 dilutions. The epitope recognizing by the SYT (N-18) was previously mapped to the N-terminus of SYT gene. Slides were incubated with the above primary antibody for 1 h at room temperature; thereafter, secondary anti-goat antibody was applied and the reaction was developed using 3, 3′-diaminobenzidine. In addition, a limited number of synovial sarcoma cases were stained with a SSX antibody (N-18, catalog sc-8816, Santa Cruz Biotechnology).
Morphologic Evaluation
A dark brown nuclear staining was considered to be positive for SYT-N18 or SSX-C18. The pattern of staining was determined to be either diffuse, or focal. The intensity was then scored semi-quantitatively as weak, moderate and strong.
Results
In the two sets of materials (conventional sections and tissue microarray), a total number of 146 cases were evaluated including: 47 synovial sarcoma and 99 other types of soft tissue tumors. Among the 47 synovial sarcoma, 12 were retrieved from the Loyola University Medical Center and 35 cases were from the tissue microarray sections obtained from the Cleveland Clinic. The staining characteristics of SYT in the conventional tissue sections of the synovial sarcoma cases are summarized in Table 1. All of the cases analyzed in the conventional sections showed strong nuclear staining, including nine MSS, two BSS and one poorly differentiated synovial sarcoma. In the BSS, both the epithelial and the spindle cell component of the tumor cells showed a similar extent and intensity of SYT staining.
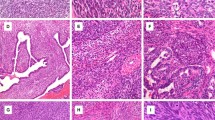
The SYT staining pattern in tissue microarray sections are summarized in Table 2. Forty-one of 47 (87%) synovial sarcoma cases from both conventional tissue and tissue microarray sections showed SYT nuclear staining (Figure 1) (Table 3). Among the non-synovial sarcoma soft tissue tumors, only 19 of 101 (19%) cases showed variable weak nuclear and/or cytoplasmic staining with the SYT antibody. Interestingly, five of 25 malignant peripheral nerve sheath tumor showed variable degrees of nuclear SYT expression. This staining appeared to be weaker in intensity compared with the synovial sarcoma cases involving up to 60% of the tumor cells. In addition, four of 17 GIST and two of two epithelioid sarcoma/PNET showed weak cytoplasmic expression of SYT in up to 20% of the tumor cells. We have noted the samples that are processed recently (up to 2 years) tends to give a brighter and more crisp staining pattern in contrast to aged tissue samples stained for the SYT protein.
Histologic and immunohistochemical staining analysis of SYT in synovial sarcomas: (a and b) monophasic synovial sarcoma (c and d) biphasic synovial sarcoma (hematoxylin and eosin staining, × 100 and × 400 magnifications). Immunostaining of SYT in monophasic synovial sarcoma (e and f) and in biphasic synovial sarcoma (g and h) illustrating typical pattern of distinct SYT nuclear expression.
Frozen and formalin fixed cell blocks of synovial sarcoma cell line (BAC) containing the SYT–SSX fusion gene also showed variable nuclear staining for SYT and SSX1 antibodies (not shown). In addition, all of 12 conventional tissue sections of synovial sarcoma showed nuclear expression of SSX1 (not shown). However, as discussed below, as SSX1 was expressed in many other tumor types, we elected not to further investigate the expression pattern in other spindle cell neoplasms, as it is unlikely to be used for a differential diagnostic work-up.
Discussion
Our study shows that antibodies directed against the SYT protein can detect over 85% of synovial sarcoma in tissue sections. The staining pattern in synovial sarcoma is exclusively nuclear, diffuse and of high intensity and contrasts with the weak, variable nuclear or cytoplasmic staining that is encountered in a small percentage (19%) of non-synovial sarcoma soft tissue tumors. This is the first study analyzing differential expression of SYT in spindle cell neoplasms using paraffin-embedded tissue sections. To our knowledge, there has been only a single study using a polyclonal antibody against SYT–SSX2 to detect the SYT–SSX fusion protein in synovial sarcoma.4 Hashimoto et al previously developed two polyclonal antibodies against the N-terminal and C-terminal portion of the SYT–SSX2 fusion protein that recognized a 61-kDa single protein in the immunoprecipitate of cell lysates from the kidney fibroblast COS-7 transfected with SYT–SSX1 and SYT–SSX2 fusion genes. These antibodies were also used to detect a native protein of 61 kDa in the lysate of a human synovial sarcoma cell line with immunoprecipitation, and in the extracts of human synovial sarcoma tissues with Western blot analysis. The antibody against the C-terminal portion of the SYT–SSX2 fusion protein was used for immunohistochemical analysis on frozen sections of three synovial sarcoma patients, which demonstrated variable immunostaining in tumor cell nuclei.4
SSX protein expression in tissues has a profile similar to cancer testis antigens, and it has various expressions in several different types of tumors.8, 9 Given this background, we focused primarily on the expression pattern of SYT rather than SSX, as it seemed likely that SSX protein expression is not specific for synovial sarcoma. This fact was further supported by another recent study that demonstrated a high level expression of SSX mRNA in some malignant bone tumors (osteosarcomas) and non-synovial sarcoma soft tissue tumors.14 In our preliminary immunoanalysis of non-synovial sarcoma soft tissue tumors, we observed that the SSX1 antibody did not distinguish synovial sarcoma from other common spindle cell neoplasms (data not shown).
Several studies have demonstrated that SYT, SSX proteins and SYT–SSX fusion proteins to be localized in the nucleus of tumor cells of synovial sarcoma cell lines.10, 11, 15 The N-terminus of SYT determines its nuclear localization and the 34 amino acids of C-terminus (also called SSXRD domain) mediates SSX nuclear localization. The SSXRD domain also plays a pivotal role in determining the subnuclear localization of the SYT–SSX chimeric oncoprotein. The SYT–SSX fusion proteins contain both potential transcription activating and repressing domains and may as a transcriptional regulator through interaction with other proteins. These oncoproteins may deregulate transcription through changes in chromatin structure and interference with chromatin remodeling to trigger sarcomagenesis.8, 10 Studies targeting SYT or SYT–SSX fusion proteins in synovial sarcoma may provide a novel approach for clinical treatment of these neoplasms.16
This is the first study providing tissue-based evidence that the SYT and SSX proteins are expressed in the nucleus of synovial sarcoma cells. We also found that tissue sections from recently diagnosed synovial sarcoma showed a stronger immunoreactivity and less background stains than that of tissue sections from archived cases. Various factors can influence immunoreactivity patterns, including the protein integrity and the type of fixative used.17 In archival paraffin-embedded blocks, there tends to be a progressive loss of the structural integrity of proteins. This could partly explain the variable intensity of immunoreactivity in some of the synovial sarcoma cases analyzed.
Thirteen of 99 cases (13%) of non-synovial sarcoma soft tissue tumors cases demonstrated variable nuclear staining of SYT protein in neoplastic cells. Possible explanations for this staining in the non-synovial sarcoma soft tissue tumors may include nonspecific staining, technical inaccuracy (eg, insufficient antigen retrieval), or this could represent true SYT protein expression in some non-synovial sarcoma soft tissue tumors.
Interestingly, among the 13 non-synovial sarcoma soft tissue tumor cases that showed nuclear SYT staining, five were malignant peripheral nerve sheath tumor, with a variable nuclear staining intensity ranging from 20 to 60% of the tumor cells. Interestingly, not only synovial sarcoma and malignant peripheral nerve sheath tumor share certain common histologic features, a recent study comparing gene-expression profiles using cDNA microarray demonstrated that synovial sarcoma has a gene-expression pattern that is distinct from other sarcomas, but is strikingly similar to malignant peripheral nerve sheath tumors.18 Contrarily, a study by Nielsen et al19 analyzing gene-expression patterns of 41 soft-tissue tumors with spotted cDNA microarrays found that synovial sarcoma, GIST, some neural tumors and a subset of the leiomyosarcomas, showing strikingly distinct gene-expression patterns.
Currently, a panel of markers including cytokeratin, EMA, CD99 and bcl-2 is often utilized to aid in the diagnosis of synovial sarcoma. However, each of these markers can be expressed in a variety of other tumors. A recent study20 used cluster analysis to analyze immunohistochemical data obtained from a tissue microarray of 73 soft tissue tumors (23 synovial sarcoma, 23 malignant peripheral nerve sheath tumor and 27 Ewing/PNET) with 22 different antibodies, including cytokeratin, CD99, EMA and bcl-2. The data suggested two clusters among synovial sarcoma, one cluster (n=11) was cytokeratin and EMA positive and another (n=9) was cytokeratin negative, but EMA, bcl-2 and mostly CD56 positive. The study also highlighted aberrant staining reactions and diagnostic pitfalls in these tumors.20 We believe the use of practical immunostaining assay for the SYT detection is likely to decrease the number of cases requiring large battery of studies for precise diagnosis of synovial sarcoma. If the staining shows an equivocal results or the case is histologically suspicious for a lesion other than synovial sarcoma such as a malignant peripheral nerve sheath tumor, various ancillary studies including molecular assays and electron microscopy could be performed as usual for further work-up. We believe the addition of the SYT antibody in the diagnostic immunohistochemical panel will allow the pathologist an even higher level of diagnostic accuracy, when evaluating spindle cell tumors of soft tissue.
The t(X; 18)(p11.2; q11.2) translocation with SYT–SSX fusion genes represents the most characteristic feature of synovial sarcoma, and can be detected by FISH or RT-PCR techniques in fresh and frozen tumor tissues, as well as formalin-fixed, paraffin-embedded tumor tissues.21, 8, 2 However, laboratories with limited resources will unlikely use these approaches as molecular assays require trained individuals and tend to be relatively sophisticated and time consuming. The widespread use of each of IHC provides a significant advantage to the diagnostic pathologist and as such we believe SYT immunostain to be an extremely useful adjunct to the diagnostic armamentarium. In conclusion, this is the first study demonstrating expression of SYT in tissue sections of synovial sarcoma and indicates that this method could provide an easy, rapid and widely applicable means of assisting in the diagnosis of synovial sarcoma, particularly when material and/or resources are unavailable for PCR or FISH-based testing.
References
Weiss SW, Goldblum JR . Enzinger and Weiss's Soft Tissue Tumors, 4th edn. Mosby: Philadelphia, 2001.
Kawauchi S, Fukuda T, Chochi Y, et al. Reverse transcription-polymerase chain reaction in situ hybridization for SYT-SSX fusion gene transcripts in synovial sarcomas. Int J Mol Med 2005;16:763–766.
Xu Z, Yang T, Wu B, et al. Detection and analysis of SYT–SSX fusion gene in synovial sarcoma. Zhonghua Bing Li Xue Za Zhi 2001;30:431–433.
Hashimoto N, Araki N, Yoshikawa H, et al. SYT–SSX fusion proteins in synovial sarcomas: detection and characterization with new antibodies. Cancer Lett 2000;149:31–36.
Clark J, Rocques PJ, Crew AJ, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet 1994;7:502–508.
Limon J, Dal Cin P, Sandberg AA . Translocations involving the X chromosome in solid tumors: presentation of two sarcomas with t(X;18)(q13;p11). Cancer Genet Cytogenet 1986;23:87–91.
van de Rijn M, Barr FG, Collins MH, et al. Absence of SYT–SSX fusion products in soft tissue tumors other than synovial sarcoma. Am J Clin Pathol 1999; 112:43–49.
dos Santos NR, de Bruijn DR, van Kessel AG . Molecular mechanisms underlying human synovial sarcoma development. Genes Chromosomes Cancer 2001;30:1–14.
Dos Santos NR, Torensma R, de Vries TJ, et al. Heterogeneous expression of the SSX cancer/testis antigens in human melanoma lesions and cell lines. Cancer Res 2000;60:1654–1662.
dos Santos NR, de Bruijn DR, Balemans M, et al. Nuclear localization of SYT, SSX and the synovial sarcoma-associated SYT–SSX fusion proteins. Hum Mol Genet 1997;6:1549–1558.
Brett D, Whitehouse S, Antonson P, et al. The SYT protein involved in the t(X;18) synovial sarcoma translocation is a transcriptional activator localised in nuclear bodies. Hum Mol Genet 1997;6:1559–1564.
dos Santos NR, de Bruijn DR, Kater-Baats E, et al. Delineation of the protein domains responsible for SYT, SSX, and SYT-SSX nuclear localization. Exp Cell Res 2000;256:192–202.
Nagai M, Tanaka S, Tsuda M, et al. Analysis of transforming activity of human synovial sarcoma-associated chimeric protein SYT-SSX1 bound to chromatin remodeling factor hBRM/hSNF2 alpha. Proc Natl Acad Sci USA 2001;98:3843–3848.
Naka N, Joyama S, Tsukamoto Y, et al. Quantification of SSX mRNA expression in human bone and soft tissue tumors using nucleic acid sequence-based amplification. J Mol Diagn 2005;7:187–197.
Soulez M, Saurin AJ, Freemont PS, et al. SSX and the synovial-sarcoma-specific chimaeric protein SYT-SSX co-localize with the human Polycomb group complex. Oncogene 1999;18:2739–2746.
Albritton KH, Randall RL . Prospects for targeted therapy of synovial sarcoma. J Pediatr Hematol Oncol 2005;27:219–222.
Ananthanarayanan V, Pins MR, Meyer RE, et al. Immunohistochemical assays in prostatic biopsies processed in Bouin's fixative. J Clin Pathol 2005;58:322–324.
Fukukawa C, Nakamura Y, Katagiri T . Molecular target therapy for synovial sarcoma. Fut Oncol 2005;1:805–812.
Nielsen TO, West RB, Linn SC, et al. Molecular characterization of soft tissue tumours: a gene expression study. Lancet 2002;359:1301–1307.
Olsen SH, Thomas DG, Lucas DR . Cluster analysis of immunohistochemical profiles in synovial sarcoma, malignant peripheral nerve sheath tumor, and Ewing sarcoma. Mod Pathol 2006;19:659–668.
Campbell C, Gallagher J, Dickinson I . Synovial sarcoma—towards a simplified approach to prognosis. ANZ J Surg 2004;74:727–731.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, R., Patel, R., Alkan, S. et al. Immunostaining for SYT protein discriminates synovial sarcoma from other soft tissue tumors: analysis of 146 cases. Mod Pathol 20, 522–528 (2007). https://doi.org/10.1038/modpathol.3800766
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800766