Abstract
Histologically, desmoplastic small round cell tumor is composed of the characteristic neoplastic small round cells with divergent differentiation, and distinct desmoplastic stroma. Genetically, the tumor shows a characteristic 11;22 translocation, involving the EWS gene on chromosome 22 and the WT1gene on chromosome 11 to produce an EWS-WT1 fusion gene which generates a chimeric protein functioning as a novel transcription factor that activates expression of target genes such as PDGF-A. Expression of PDGF-A, a potent growth factor for fibroblasts, has been detected in desmoplastic small round cell tumors and has been linked to the characteristic desmoplasia in these tumors. Bone morphogenic proteins, which are members of the TGFβ superfamily play a complex role in regulating cell growth and differentiation and bone formation but have not been evaluated in desmoplastic small round cell tumors. In all, 24 desmoplastic small round cell tumors with EWS-WT1 fusion product confirmed by RT-PCR analysis were evaluated for expression of PDGF-A, PDGF-Rβ, TGFβ3 and bone morphogenic protein-4 by standard immunohistochemical methods with antigen retrieval on paraffin sections. Immunoreactivity was evaluated semiquantitively. Tumor-associated desmoplasia was quantified using a three-tier scale on hematoxylin- and eosin-stained sections. Desmoplastic small round cell tumors showed variable immunoreactivity with TGFβ3 (21/24), BMP4 (14/21), PDGF-A (19/24) and PDGF-Rβ (16/22). Less frequently, the stromal cells showed reactivity with TGFβ3, PDGF-Rβ and PDGF-A. Tumor-associated desmoplasia was prominent in eight, intermediate in seven and weak in nine cases. There was no correlation between tumor-associated desmoplasia and the markers tested except PDGF-A. In contrast to a previous study, our study showed that the level of PDGF-A expression inversely correlated with tumor-associated desmoplasia. Other targets of the EWS-WT1 transcription factor other than PDGF-A may be directly responsible for the prominent tumor-associated desmoplasia seen in desmoplastic small round cell tumor.
Similar content being viewed by others
Main
Desmoplastic small round cell tumor is a highly aggressive tumor usually involving the abdominal peritoneum of young males.1, 2, 3 Histologically, it is composed of neoplastic small round cells with divergent differentiation in a characteristic prominent stromal proliferation called desmoplasia.1, 2, 3, 4, 5 Genetically, the tumor shows a characteristic t(11:22)(p13;q12) involving the EWS on chromosome 22 and the WT1 on chromosome 11, resulting in an EWS-WT1 fusion gene.6, 7, 8 The EWS-WT1 fusion gene generates a chimeric protein containing the potential transactivation domain of the EWS protein fused to zinc-finger DNA-binding domain of the WT1 protein. The chimeric protein encoded by the EWS-WT1 gene is postulated to lose the tumor suppressor effect of WT1 and gain the transactivation activity because of the juxtaposed EWS domain, and thereby function as a novel transcription factor that activates expression of target genes normally repressed by WT, such as platelet-derived growth factor A (PDGF-A) and transforming growth factor-β (TGF-β).9, 10, 11 Alternatively, the fusion protein may also ctivate target genes that are not regulated by wild-type WT1 due to changes in the DNA binding domain.12 A previous in vitro study has shown that PDGF-A was upregulated by EWS-WT1 in desmoplastic small round cell tumor specimens as well as in an inducible cell line.9 Expression of PDGF-A, a potent mitogen and growth factor for fibroblasts, has been speculated to play a role in the characteristic tumor-associated desmoplasia in desmoplastic small round cell tumor, the extent of which varies from case to case.7, 9 In addition, the EWS-WT1 chimeric protein has recently been shown to be a potent transcriptional activator of expression of the beta chain of the IL-2/15 receptor in tumor cells and expression of IL2 and IL-15 has been detected in the desmoplastic stroma in desmoplastic small round cell tumors.13 The prominent stromal reaction might also provide a paracrine signal to neoplastic cell proliferation in these tumors.13
Bone morphogenic proteins (BMP) are members of the TGF-β superfamily and have a complex role in regulating cell growth and differentiation including bone formation.14 Overexpression of BMP4 has been found in patients with fibrodysplasia ossificans progressiva and various bone and soft-tissue sarcomas.15, 16 Little is known about the potential role of BMP in neoplastic proliferation and stromal desmoplasia in desmoplastic small round cell tumor.
This study was performed to further evaluate the role of PDGF-A and BMP4 in regulating the tumor-associated desmoplasia in desmoplastic small round cell tumors on routine surgical specimens by evaluating the level of PDGF-A and BMP4 immunoreactivity in neoplastic cells in relation to the amount of the desmosplasia associated with these tumors.
Materials and methods
In all, 24 cases of DSRCT were included for the study, eight from Children Hospital of Philadelphia, three from Cleveland Clinic Foundation, five from Hospital of the University of Pennsylvania and eight from consultation file of one of the coauthors (FGB). EWS-WT1 fusion product was previously identified in all cases by RT-PCR using frozen tissue.17 Tissues were fixed in 10% formalin for 12–24 h and embedded in paraffin for routine histologic evaluation. Of the 24 cases, 20 were large specimens of en-bloc excision of the tumor in which a representative block containing large section of tumor tissue was available for immunohistochemical staining. The remaining four cases are excisional biopsy specimens. In addition to a representative section, multiple sections of the same tumor were also available for evaluation in 10 cases to assess heterogeneity in the distribution of tumor-associated desmoplasia in a given tumor. Sections, 4 μm thick, were pretreated in a microwave oven (1100 W) for 12 min at 70% power level in 1 × citrate buffer before incubation with primary antibodies. Primary antibodies against PDGF-A (pAb 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), PDGF-Rβ (Ab-4, clone CM95, 1:50, Oncogene Research Products, Boston, MA, USA), TGFβ3 (pAb, 1:125, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and BMP4 (mAb, Genetics Institute, Boston, MA, USA) were incubated in 4°C overnight. Streptavidin horseradish peroxidase method was used on Biotek Techmate 1000 autostainer (Ventana Medical System, Tuscan, AR, USA). Formalin-fixed, paraffin-embedded tissue of dermatofibrosarcoma protuberans, bone marrow, salivary gland, fetal brain, and cartilage were used as positive controls for PDGFA, PDGF-Rβ, TGFβ3 and BMP4 accordingly.18, 19, 20, 21, 22 A negative control was performed on each case by replacing the primary antibodies with mouse or rabbit serum. Immunoreactivity was evaluated semiquantitatively for the percentage of positive tumor cells and the intensity of the immunoreactivity (0–3+) in the neoplastic cells. A score of 0–300 was given based on the product of the percentage of the positive tumor cells and intensity of the immunoreactivity. Routine histologic sections of all cases were reviewed for semiquantitative assessment of tumor-associated desmoplasia using × 2.5 to × 5 object lenses on LEICA DMLB microscope (Leica). Based on the ratio of the amount of tumor-associated desmoplasia to neoplastic cellular component, each case was classified as desmoplasia-poor (1+, ratio <0.25), desmoplasia-moderate (2+, ratio >0.25–<1.0) or desmoplasia-rich (3+, ratio >1.0). Correlation analysis of nonparametric data was performed using GraphPad Prism Version 3.0 (GraphPad Software, Inc. San Diego, CA, USA).
Results
The majority of the tumors showed variable cytoplasmic immunoreactivity for TGFβ3 (21/24), BMP4 (14/21), PDGF-A (19/24), and PDGF-Rβ (16/22) (Table 1). Of 22 cases, 14 coexpressed PDGF-A and PDGF-Rβ. TGFβ3, PDGF-Rβ and PDGF-A immunoreactivities were also seen less frequently in stromal cells. Expression of these markers did not correlate with each other. Tumor-associated desmoplasia was rich (3+) in eight, moderate (2+) in seven and poor (1+) in nine cases. The amount of tumor-associated desmoplasia varied slightly in different areas on a given section as well as different sections of a given case on low magnification. However, given the method used for the assessment, this mild heterogeneity did not have any impact on the overall score of the tumor-associated desmoplasia even in cases in which multiple sections of the tumor were reviewed. Of 17 tumors, 15 with low level of PDGF-A immunoreactivity (score=0 or <100) were desmoplasia-rich (3+) and desmoplasia-moderate (2+) cases (Figure 1). Four of the eight desmoplasia-rich (3+) cases were completely negative for PDGF-A (score 0). In contrast, seven tumors with high level of PDGF-A (score=or >100) were all desmoplasia-poor cases (1+) (Figures 1 and 2). There was a significant inverse correlation between the amount of tumor-associated desmoplasia and the level of PDGF-A expression (P=0.0004, Graph 1). However, there was no significant correlation between desmoplasia and PDGF-A expression when comparison was made between desmoplasia-rich (3+) and desmoplasia-moderate (2+) cases (P=0.108). There was no significant correlation between the amount of desmoplasia and the level of the immunoreactivity of the other markers tested (P>0.1). Results are summarized in Table 1.
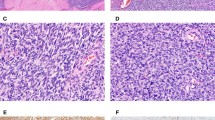
Extent of the desmoplasia (A–C) and the corresponding PDGF-A immunoreactivity (a, b and c) in desmoplasia-poor, moderate and rich desmoplastic small round cell tumors. (A) and (a) Strong PDGFA in a desmoplasia-poor tumor (case 18). (B) and (b) Focal PDGF-A in a desmoplasia-moderate tumor (case 12). (C) and (c) lack of PDGF-A in a desmoplasia-rich tumor (case 3). (A–C) Hematoxylin and eosin staining; (a, b and c) Immunoperoxidase staining).
Higher magnification of images (a) and (b) in Figure 1.

Graph 1
Discussion
Because of its role as a growth factor for fibroblasts, the expression of PDGF-A induced by the EWS-WT1 chimeric protein has been speculated to induce the formation of the desmoplastic stroma in desmoplastic small round cell tumors.9 Thus, high level of PDGF-A expression would be expected in desmoplasia-rich desmoplastic small round cell tumors. In this study, we evaluated immunoreactivity of PDGF-A, PDGF-Rβ, BMP4 and TGFβ3 and correlated their expression with the amount of tumor-associated desmoplasia and found that only the level of PDGF-A immunoreactivity was related to the extent of desmoplasia in desmoplastic small round cell tumors. However, in contrast to previous speculation, our study showed that the level of PDGF-A immunoreactivity was apparently inversely correlated with desmoplasia in desmoplastic small round cell tumors.9 Desmoplasia-rich tumors tended to have lower levels of PDGF-A, while desmoplasia-poor tumors tended to have higher levels of PDGF-A, as determined by immunohistochemistry. This inverse correlation between PDGF-A and tumor-associated desmoplasia is poorly understood and merits further investigation. It might also suggest that the effect of PDGF-A on formation of desmoplasia in desmoplastic small round cell tumors is complex and indirect. Other targets of the EWS-WT1 transcription factor other than PDGF-A may be directly responsible for the prominent stromal desmoplasia seen in this tumor.
Strong immunoreactivity of TGF-β1 and PDGF-Rα was previously reported in desmoplastic small round cell tumors.23 Although we have also found frequent TGFβ3, PDGF-Rb and BMP4 immunoreactivity in these tumors, expression of these proteins did not quantitatively correlate with the amount of tumor-associated desmoplasia and, therefore, is unlikely to play a critical role in the formation of desmoplasia in desmoplastic small round cell tumors.
In summary, among the growth factors tested, only PDGF-A was significantly correlated with tumor-associated desmoplasia. Contrary to previous speculation, we found an inverse relationship between PDGF-A immunoreactivity and amount of desmoplasia in desmoplastic small round cell tumors. This finding suggests the role of PDGF-A in desmoplastic small round cell tumors is more complex than previously expected. TGF-β3 and BMP4 are known to have complex effects on regulating tissue proliferation and differentiation, but do not seem to play a critical role in inducing stromal proliferation in these tumors. Because PDGF-Rβ normally binds PDGF-BB as well as AB chains, coexpression of PDGF-A and PDGF-Rβ might have an autocrine effect in desmoplastic small round cell tumors.23, 24, 25
References
Gerald WL, Rosai J . Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol 1989;9:177–183.
Gonzalez-Grussi F, Crawford SE, Sun CCJ . Intraabdominal desmoplastic small-cell tumors with divergent differentiation. Am J Surg Pathol 1992;14:633–642.
Gerald WL, Miller HK, Battifora H, et al. Intra-abdominal desmoplastic small round-cell tumor. Am J Surg Pathol 1991;15:499–513.
Ordonez NG, Zirkin R, Bloom RE . Malignant small-cell epithelial tumor of the peritoneum coexpressing mesenchymal-type intermediate filaments. Am J Surg Pathol 1989;13:413–421.
Ordonez NG, El-Naggar AK, Ro JY, et al. Intra-abdominal thermoplastic small cell tumor. Hum Pathol 1993;24:850–865.
Sawyer JR, Tryka AF, Lewis JM . A novel reciprocal chromosome translocation t(11;22)(p13;q12) in an intraabdominal desmoplastic small round-cell tumor. Am J Surg Pathol 1992;16:411–416.
Gerald WL, Ladanyi M, de Alava E, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11,22)(p13;q12): desmoplastic small round cell tumors. J Clin Oncol 1998;16:3028–3036.
Chan As, MacNeill S, Thorner P, et al. Varian EWS-WT1 chimeric product in the desmoplastic small round cell tumor. Pediatr Dev Pathol 1999;2:188–192.
Bong Lee S, Kolquist KA, Nichols K, et al. The EWS-WT1 translocation product induces PDGFA in desmoplastic small round-cell tumour. Nat Genet 1997;17:309–313.
Wang Z, Madden S, Deuel T, et al. The Wilms’ tumor gene product WT1, represses transcription of the platelet-derived growth factor A-chain gene. Biol Chem 1992;265:21999–22002.
Gashler AL, Bonthron DT, Madden SL, et al. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms’ tumor suppressor WT1. Proc Natl Acad Sci USA 1992;89:10984–10988.
Kim J, Lee K, Pelletier J . The DNA binding domains of the WT1 tumor suppressor gene product and chimeric EWS/WT1 oncoprotein are functionally distinct. Oncogene 1991;16:1021–1030.
Wong JC, Lee SB, Bell MD, et al. Induction of the interleukin-2/15 receptor beta-chain by the EWS-WT1 translocation product. Oncogene 2002;21:2009–2019.
Miyazono K, Kusanagi K, Inoue H . Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol 2001;187:265–276.
Shore EM, Xu M, Shah PB, et al. The human bone morphogenetic protein 4 (BMP-4) gene: molecular structure and transcriptional regulation. Calcified Tissue Int 1998;63:221–229.
Yoshikawa H, Rettig WJ, Lane JM, et al. Immunohistochemical detecton of bone morphogenetic proteins in bone and soft-tissue sarcomas. Cancer 1994;74:842–847.
Barr FG, Chatten J, D’Cruz CM, et al. Molecular assays for chromosomal translocations in the diagnosis of pediatric soft tissue sarcomas. JAMA 1995;273:553–557.
Palman C, Bowen-Pope DF, Brooks JJ . Platelet-derived growth factor receptor (beta-subunit) immunoreactivity in soft tissue tumors. Lab Invest 1992;66:108–115.
McCune BK, Patterson K, Chandra RS, et al. Expression of transforming growth factor-beta isoforms in small round cell tumors of childhood. An immunohistochemical study. Am J Pathol 1993;142:49–58.
Taniuchi K, Yamada Y, Nonomura A, et al. Immunohistochemical analysis of platelet-derived growth factor and its receptors in fibrohistiocytic tumors. J Cutaneous Pathol 1997;24:393–397.
Liu HM, Yang HB, Chen RM . Expression of basic fibroblast growth factor, nerve growth factor, platelet-derived growth factor and transforming growth factor-beta in human brain abscess. Acta Neuropathol 1994;88:143–145.
Schmits B, Wicherhauser C, Thiele J, et al. Megakaryocyte induced fibroblast proliferation is enhanced by costimulation with IL-6/IL-3 and dependent on secretory and adhesion events. Leuk Res 1999;23:723–729.
Froberg K, Brown RE, Gaylord H, et al. Intra-abdominal desmoplastic small round cell tumor: immunohistochemical evidence for up-regulation of autocrine and paracrine growth factors. Ann Clin Lab Sci 1999;29:78–85.
Kanakaraj P, Raj S, Khan SA, et al. Ligand-induced interaction between alpha- and beta-type platelet-derived growth factor (PDGF) receptors: role of receptor heterodimers in kinase activation. Biochemistry 1991;30:1761–1767.
Harsh GR, Keating MT, Esobedo JA, et al. Platelet derived growth factor (PDGF) autocrine components in human tumor cell lines. J Neuro-Oncol 1990;8:1–12.
Acknowledgements
This research was funded in part by NIH Grants CA89461 and CA24507 (to FGB). The authors thank Dr Jasvir S Khurana for allowing us to use the antibody against bone morphogenic protein-4 acquired from Genetics Institute, Boston, MA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, P., Goldblum, J., Pawel, B. et al. PDGF-A, PDGF-Rβ, TGFβ3 and bone morphogenic protein-4 in desmoplastic small round cell tumors with EWS-WT1 gene fusion product and their role in stromal desmoplasia: an immunohistochemical study. Mod Pathol 18, 382–387 (2005). https://doi.org/10.1038/modpathol.3800264
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800264
Keywords
This article is cited by
-
Pazopanib in advanced desmoplastic small round cell tumours: a multi-institutional experience
Clinical Sarcoma Research (2014)
-
Paratesticular desmoplastic small round cell tumour: an unusual tumour with an unusual fusion; cytogenetic and molecular genetic analysis combining RT-PCR and COBRA-FISH
Clinical Sarcoma Research (2012)
-
Multicenter Phase II trial assessing effectiveness of imatinib mesylate on relapsed or refractory KIT-positive or PDGFR-positive sarcoma
Journal of Orthopaedic Science (2010)