Abstract
Decreased expression of p27 (a cyclin-dependent kinase inhibitor) is an adverse prognostic marker in a diverse array of human cancers. The purpose of this study was to investigate the expression of p27 and Jab1 (a protein involved in p27 degradation) in melanocytic lesions, and to identify their possible participation in melanoma progression. A tissue microarray was constructed using formalin-fixed, paraffin-embedded archival tissue blocks of 94 melanocytic lesions including 19 benign nevi, 21 dysplastic nevi, 23 melanomas, and 31 metastatic melanomas. The expression of p27 and Jab1 was evaluated by immunohistochemistry. The association between p27, Jab1, and clinicopathological parameters was analyzed using χ2 and Fisher's exact tests. Nonparametric Pearson's rank correlation was applied to evaluate the relationship between p27 and Jab1 expression. p27 was expressed in 15 (88%) nevi, 18 (95%) dysplastic nevi, 11 (50%) melanomas, and only in four (13%) of the metastatic melanomas (P<0.001). Jab1 was expressed in 14 (82%) standard nevi, 18 (95%) dysplastic nevi, 17 (77%) melanomas, and 16 (53%) of the metastatic melanomas (P<0.01). In metastatic melanomas, there was a negative correlation between p27 and Jab1 expression (r=−0.166). The low levels of p27 in primary and metastatic melanoma cases may explain the high proliferation rate of such lesions. Also, the relative high expression of Jab1 in metastatic melanoma, associated with low levels of p27, suggests that Jab1 may be involved in survival and proliferation of metastatic melanoma cells.
Similar content being viewed by others
Main
Cell proliferation consists of a series of biochemical events primarily maintained by the activity of the serine/threonine kinases known as cyclin-dependent kinases (cdk) that are under the strict control of positive and negative regulators.1, 2 The function of cdk is regulated by phosphorylation and dephosphorylation of the catalytic subunit, and by association with activating subunits called cyclins. The cdk inhibitors suppress the activity of the cyclin/cyclin-dependent kinase complexes.1, 2, 3 Disruption of the cell cycle control is considered a hallmark of tumorigenesis and plays an important role in tumor progression.1, 4
There are two major families of cdk inhibitors, one being the Ink4 family and the other is Cip/Kip family. p27Kip1 (p27) belongs to the Cip/Kip family and is a critical, universal cdk-inhibitory protein. p27 suppresses the G1-to-S cell cycle progression, functions as a major negative regulator of apoptosis and is, therefore, considered a tumor suppressor.1, 5, 6 When located in the nucleus, p27 is active and effectively inhibits cdk. Upon mitogenic stimuli, a fraction of p27 is translocated from the nucleus in the cytoplasm, reducing its nuclear concentration and the cells proliferate under unrestricted cdk activity. Although the mechanism underlying p27 deregulation in human cancer is poorly understood, several studies have shown that p27 is an independent prognostic marker in many human cancers.1, 7 It has been generally accepted that decreased p27 expression correlates with higher tumor grade and poor survival rates among patients with tumors of different histogenesis including, lung, colon, breast, and ovarian carcinomas.8, 9, 10, 11, 12 Some tumors (eg, colon, esophagus, endometrial carcinomas) show increased p27 expression when compared with normal tissues, thus suggesting the existence of alternate mechanisms blocking the p27 inhibitory function over cellular proliferation.13, 14, 15, 16
There are several possible mechanisms involved in cancer-associated abnormalities of p27 expression.1, 17, 18 It has been shown that, although p27 protein is downregulated in human tumors, p27 mRNA is not, eliminating the possibility of transcriptional mechanisms as the cause of this decrease.1 Also, mutations of the gene encoding p27 are rare, suggesting that alterations in mechanisms regulating its degradation are more likely to be involved.1, 17 It has recently been demonstrated that p27 degradation is accelerated by the c-Jun activation domain-binding protein-1 (Jab1).18, 19 Jab1 is a subunit of a large protein complex called COP9 signalosome, originally identified as a coactivator of the AP1 transcription factor, and involved in the control of cell proliferation.20 Jab1 also causes translocation of p27 from the nucleus to cytoplasm, decreasing the p27 nuclear level and, thereby accelerating its degradation via the ubiquitin–proteasome pathway.17, 21 Recent studies have shown an inverse correlation of Jab1 and p27 expression levels in a diverse array of human neoplasms and raised the hypothesis that detection of this correlation might be helpful in predicting tumor biological behavior.22, 23, 24, 25
The p27 expression has recently been investigated in melanocytic lesions.5, 26, 27, 28, 29, 30 However, the status of Jab1 expression in these lesions, including its possible clinical significance and the correlation with p27 is still unknown.
The purpose of this study was to investigate the level of expression and correlation of p27 and Jab1 in various melanocytic lesions, and to identify their potential significance for diagnosis and prognosis of such lesions.
Materials and methods
Patients
The pathology database at the University of Texas MD Anderson Cancer Center was retrospectively reviewed and we randomly selected cases of benign and malignant melanocytic lesions diagnosed in our institution between 1 January 2000 and 31 December 2001. There were identified 94 patients with various melanocytic lesions including 19 benign nevi, 21 dysplastic nevi, 23 melanomas (13 nodular/acral/lentigo melanomas, 10 superficial spreading melanomas), and 31 cases of metastatic malignant melanomas (12 cases of subcutaneous metastasis, 10 cases of visceral metastasis, and nine cases of lymph node metastasis). The pathology reports and the hematoxylin and eosin (H&E)-stained glass slides were reviewed.
Construction of Tissue Microarray
Tissue microarray analysis was recently validated for histological study using immunohistochemistry (IHC). In the present study, we have followed the established criteria regarding tissue microarray construction, thus reducing the problem of lesion heterogeneity, in order to obtain concordant results between tissue microarrays and full section analysis.31
Three tissue microarrays containing 94 melanocytic lesions were constructed using formalin-fixed, paraffin-embedded archival tissue blocks and a manual tissue microarrayer (Beecher Instruments, Silver Spring, MD, USA). The H&E-stained tissue sections from donor blocks were screened for the representative and optimal areas. Tissue cores with a diameter of either 0.6 or 1.0 mm (depending on the lesion size) were sampled and arrayed on a recipient paraffin block. All of the melanocytic lesions were sampled in duplicate except five cases of benign nevus, which were sampled with a single 0.6 mm diameter due to the small size of the specimen. Two control cases (one benign nevus and one malignant melanoma) were included in all three microarray blocks to serve as baseline controls. In all, 5 μm sections of the tissue array blocks were cut, placed on glass slides, and used for immunohistochemical analysis.
Immunohistochemical Staining for p27 and Jab1
Sections from the tissue microarrays were deparaffinized and rehydrated in graded alcohols. Antigens were retrieved using Target Retrieval Solution (DAKO Corp., Carpinteria, CA, USA), and endogenous peroxidase activity was blocked using 3% hydrogen peroxide. After incubation with protein block solution (DAKO Corp., Carpinteria, CA, USA) for 15 min to reduce nonspecific binding, the slides were incubated with primary antibody diluted with 1% bovine serum albumin in phospate-buffered saline (PBS) at room temperature for 1 h. Biotin-conjugated secondary antibody was applied for 20 min, and streptavidin–biotin–peroxidase complex (Strept-AB complex, dilution 1:200, DAKO Corp., Carpinteria, CA, USA) was added for 20 min. 3,3′-diaminobenzidine tetrahydrochloride was used as a chromogen, and hematoxylin was used as a counterstain. 4D11D8 monoclonal antibody (Zymed, San Francisco, CA, USA) diluted 1:400 was used to detect Jab1 expression, and clone SX53G8 monoclonal antibody (DAKO Corp.) diluted 1:200 was used to detect p27.
The distribution of the immunoreactivity was analyzed by quantifying nuclear staining. A consensus between the investigators (DI and VGP) was obtained to score the nuclear staining of the p27 and Jab1 as follows: 0 for less than 5% positive nuclei; 1 for 5–25% positive nuclei, 2 for 26–75% positive nuclei, and 3 for over 75% positive nuclei. Positivity of cells was defined regardless of staining intensity. By convention, we considered that greater than 25% positive cells represented the cutoff between negativity and positivity of the p27 and Jab1 immunostainings. The labeling index (LI) was defined as the percentage of immunolabeled cells. The investigators independently reviewed and scored slides by estimating the percentage of cells exhibiting characteristic nuclear staining. At least 10 high-power fields were chosen randomly, and 100 cells were counted in each field. Immunoreactive cells were counted randomly over junctional and deep dermal foci; intraepidermal melanocytes were also considered. Interobserver variation was addressed by averaging the individual values. However, the variations among investigators were identified in less than 10% of the cases and were not greater than one score group. The presence or absence of p27 and/or Jab1 cytoplasmic labeling was also recorded.
Tissue damage during the cutting and staining procedures is a phenomenon sometimes associated with the tissue microarray technique.31 From the 94 original cases, not enough tissue remained on the microarray slides for six cases and these were excluded from the study. Therefore, 88 melanocytic lesions were finally evaluated for p27 and Jab1 expression, including 17 benign nevi, 19 dysplastic nevi, 22 melanomas (13 nodular/acral/lentigo melanomas, nine superficial spreading melanomas), and 30 cases of metastatic malignant melanomas (12 cases of subcutaneous metastasis, 10 cases of visceral metastasis, and eight cases of lymph node metastasis).
Statistical Analysis
The association between p27 and Jab1 and clinicopathological parameters was analyzed using χ2 and Fisher's exact tests, with P<0.05 considered to be statistically significant. The nonparametric Pearson's rank correlation coefficient was applied to evaluate whether there was a positive or negative correlation between p27 and Jab1 expression and the strength of their relationship.
Results
p27 was strongly expressed in normal epidermal melanocytes, supporting the hypothesis that one important function of p27 may be to regulate quiescence in cells, as the normal epidermal melanocytes are generally quiescent.32 In normal or uninvolved skin adjacent to melanocytic tumors, p27 was also strongly expressed in differentiating keratinocytes localized in the upper spinous and granular layers of the epidermis; it was not detected in areas of active proliferation (basal layer). The cells situated within the dermal papilla and inner pilar sheath were positive for p27. Within the dermis, endothelial cells, isolated fibroblasts and lymphocytes also expressed p27. No Jab1 expression was detected in normal structures of the skin.
Expression of p27 in Benign and Malignant Melanocytic Lesions (Table 1)
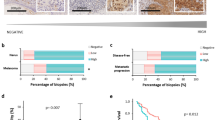
In standard nevi, p27 was strongly expressed in more than 75% of cells (score 3) in 14 out of 17 cases (Figure 1a). Notably, both cases with less than 25% of the cells expressing p27 were blue nevi (one standard blue nevus and one cellular blue nevus). Neither blue nevi had mitotic figures or necrosis suggesting an aggressive behavior. The neurotized components of the standard nevi did not express p27. The melanocytic multinucleated giant cells, occasionally present in the dermal component of nevi, were strongly p27-positive.
Examination of p27 immunostaining of 19 dysplastic nevi (Figure 1b) revealed strong positivity (score 3) in 17 cases and only one case (combined dysplastic and blue nevus) did not express p27.
In contrast, in only three of the 22 cases of melanoma, p27 expression was identified in more than 75% of cells. In all, 11 primary malignant melanomas had less than 25% of the cells expressing p27 (Figure 1c). We found no significant difference in p27 labeling index among different histologic types of melanoma. The percentage of p27-positive cells was significantly reduced in metastatic melanomas; only four cases out of 30 were p27-positive (Figure 1d).
In summary, considering 25% of positive cells as cutoff, p27 was positive in 15 (88%) standard nevi, 18 (95%) dysplastic nevi, 18 (50%) melanomas, and only in four (13%) of the metastatic melanomas. Therefore, we identified a statistically significant (P<0.001) reduction of p27 expression in primary and metastatic melanomas comparing with nevi.
Regarding cytoplasmic labeling for p27 (Figure 2), it was seen in four (18%) out of 22 melanomas, and in 11 (37%) of the 30 metastatic melanomas, especially in cases negative for nuclear p27 (P<0.01). For instance, out of 26 nuclear p27-negative-metastatic melanomas, 11 (42%) had positive cytoplasmic staining.
Expression of Jab1 in Benign and Malignant Melanocytic Lesions (Table 2)
Analyzing the 17 cases of standard nevi, Jab1 was expressed in 14 (88%) cases (Figure 3a). The large, multinucleated cells seen in the dermal component of nevi, did not express Jab1. Out of 19 dysplastic nevi, 18 (95%) expressed Jab1 (Figure 3b) and 11 cases were strongly Jab1-positive (score 3).
In contrast with p27 expression, the majority of the primary melanoma cases (77%) expressed Jab1 (Figure 3c). There was no significant difference in the distribution of Jab1 labeling index among different histologic types of melanoma. In metastatic melanomas, 16 of the 30 (53%) cases expressed Jab1 (Figure 3d).
Cytoplasmic immunoreactivity for Jab1 was also noted in 17 (20%) of the 88 examined cases, with approximately equal distribution among the benign nevi, melanoma and metastatic melanoma cases. We found that in primary and metastatic melanomas, Jab1 cytoplasmic staining was present only in p27-nuclear negative cases. Out of 37 p27-negative primary and metastatic melanomas, 11 (30%) had Jab1 cytoplasmic staining.
Correlation Between p27 and Jab1 Expression (Table 3)
The correlation between Jab1 and p27 expression, as continuous variables, was investigated using Pearson's rank correlation. We identified a positive correlation of p27 and Jab1 labeling index in standard nevi, dysplastic nevi, and primary melanomas (correlation coefficient r=−0.52, 0.75, and 0.33, respectively). Both proteins were expressed in 14 out of 17 (82%) standard nevi, in 18 out of 19 (95%) dysplastic nevi, and in 10 out of 22 (45%) primary melanoma cases. p27 and Jab1 expressions were inversely correlated in metastatic melanomas (correlation coefficient r=−0.166). In these cases, a high labeling index for Jab1 was correlated with a low expression of p27. More specifically, 13 out of 16 Jab1-positive metastatic melanoma cases were also p27-negative and only three cases were positive for both proteins. Notably, in benign melanocytic lesions, there were no Jab1-positive/p27-negative cases (P<0.0001).
Discussion
The mechanisms involved in cutaneous malignant melanoma progression are not fully understood. p27, a member of the universal cdk inhibitor family, has been the subject of recent studies. p27 gene was described as a putative tumor suppressor, and significantly decreased expression of p27, compared with that of a normal adjacent tissue, was reported in several human cancers.1 Overexpression of cdk-inhibitor p27 was also proved a major negative regulator of apoptosis in different tumor types.1, 5, 6
A large number of studies have shown that low levels of p27 expression are associated with poor survival rates in various human cancers,8, 9, 10, 11, 12 but not all authors have found such a relationship.13, 14, 15, 16, 25, 33, 34
In agreement with previous reports, we identified that p27 was highly expressed in normal skin melanocytes and benign nevi, supporting the notion that one important function of p27 may be to regulate cell quiescence.30 It has been shown that p27 is increased in quiescent cells as a result of mitogen deprivation, antimitotic signals, or contact inhibition, and its levels are reduced when cells are stimulated to enter the cell cycle.1, 35 Melanocytic nevi represent melanocytic clones that have undergone expansion, followed by growth-arrest and apoptosis resistance, possibly cell senescence.30 This concept is supported by the limited proliferative capacity of nevus cells, and probably explains the strong p27 expression in nevi included in our study. However, the fact that a number of the nevi did not express p27 probably indicates that p27 pathway is not the only mechanism involved in nevus cell quiescence.
We have found that p27 immunolabeling was positive in majority of standard and dysplastic nevi, in half of melanoma cases, and four (13%) of the metastatic melanomas (P<0.001). As previously described, the reduction of p27 nuclear expression in melanomas is partially explained by its translocation into the cytoplasm and increased degradation in highly active mitotic cells.1, 28, 30 Moreover, we have identified a statistical significant increase of p27 cytoplasmic expression in primary and metastatic melanomas (P<0.01), especially in cases with negative nuclear p27, demonstrating a cytoplasmic translocation of p27 translocation in these cases. For instance, out of 26 nuclear p27-negative metastatic melanomas, 11 (42%) had positive cytoplasmic expression. This finding sustains the hypothesis that sequestration of p27 in the cytoplasm blocks p27 activity, its nuclear expression, and is likely to play an important role in promoting oncogenesis.1 There are also reports showing that p27 expression in the cytoplasm rather than in nuclei is correlated with tumor recurrence and decreased survival.35, 36, 37
In a recent study, Morgan et al26 showed that analysis of p27 expression in nevi and melanomas (total 63 melanocytic lesions) failed to show any different pattern of immunostaining between benign and malignant melanocytic lesions. However, in their study they did not include any metastatic melanomas. Zhang et al27 analyzed cutaneous melanoma cell lines from matched primary and metastatic melanomas and found that p27 was expressed in most primary melanoma cell lines, but lost or reduced in their matched metastases. Our study confirms their findings by identifying a reduction of p27 expression in metastatic melanomas compared with primary melanomas (P<0.01). A study of p27 protein expression in primary colorectal carcinomas and their metastatic foci suggested that downregulation of p27 in circulating tumor cells may confer the ability to grow in an environment of altered extracellular matrix or intercellular adhesion properties that may facilitate tumor metastasis.38 Thus, we can reasonably speculate that reduced expression of p27 in metastatic melanomas might be involved in tumor progression and associated with metastatic potential. Therefore, detection of low levels of p27 may become a useful predictor of aggressive primary melanomas, as suggested in a recent study.30
It has been shown that disruption of p27 regulatory mechanisms may contribute to malignant tumoral transformation.1, 28 Jab1, a negative regulator of p27, promotes its degradation and, therefore, might contribute to the cell cycle dysregulation. Recent studies reported colocalization of p27 and Jab1 in mature cells where, for unknown reasons, Jab1 may be inactive and not bound to p27.21 This might explain the high Jab1 nuclear labeling index in standard and dysplastic nevi observed in our study, which may parallel the expression of Jab1 in proliferating lymphocytes in germinal centers.22 No Jab1 expression was detected in normal structures of the skin and this finding resembles the reported p27 positivity and lack of Jab1 expression in normal ovarian surface epithelium.12
Upon mitogenic stimulation, Jab1 causes p27 transfer to the cytoplasm and accelerates its degradation via the ubiquitin/proteasome pathway.1, 18, 22 It has been shown that Jab1 has nuclear localization in proliferating cells, but cytoplasmic Jab1 increases as p27 relocates from nucleus to cytoplasm.18, 21 Recent studies revealed that Jab1 has both cytoplasmic and nuclear localization in undifferentiated tumors comparing with a predominantly nuclear localization in differentiated tumor cells.24, 39 Significantly, in our series, we identified Jab1 cytoplasmic expression in 11 out of 36 (29%) p27-negative primary and metastatic melanoma cases. This suggests a high Jab1 turnover and that melanoma progression might be regulated, at least in part, by increased expression of Jab1 and decreased p27.
Furthermore, it has been shown that there is an inverse correlation of Jab1 and p27 levels in some human neoplasms and that analysis of their expression might be helpful in predicting their biological behavior.22, 23, 24, 25
To the best of our knowledge, there are not any published reports regarding Jab1 expression in melanocytic lesions. In the present study, Jab1 nuclear immunostaining was positive in 14 (82%) standard nevi, 18 (95%) dysplastic nevi, 17 (77%) melanomas, and 15 (50%) of the metastatic melanoma cases (P<0.01). Notably, in benign melanocytic lesions, there were no Jab1-positive/p27-negative cases. In contrast, in primary and metastatic melanomas, Jab1-positive/p27-negative cases represented 60% (6/10) and, respectively, 50% (13/26) of p27-negative cases (P<0.001).
The negative correlation between p27 and Jab1 expression in metastatic melanomas suggests that Jab1 may play an important role in modulating the activity of p27 and might precede melanoma progression. However, the present study does not demonstrate an absolute Jab1 nuclear overexpression, as described in other tumors.22, 23, 24, 25 It may be reasonable to speculate that Jab1-mediated p27 degradation is only one of the mechanisms that downregulates p27 expression and that other pathways may also exist.
In summary, we have shown that p27 is highly expressed in normal melanocytes and benign nevi, and that there is a significant reduction of p27 expression in melanomas compared with nevi (P<0.001). We hypothesize that reduced expression of p27 in melanomas may be involved in tumor progression and may play a role in the metastatic potential of these tumors. Furthermore, we have shown, for the first time, that high expression of Jab1 correlates with a low levels of p27 in metastatic melanomas, suggesting that Jab1 may be involved in survival and growth of melanoma cells.
References
Bloom J, Pagano M . Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol 2003;13:41–47.
Morgan M . Principles of cdk regulation. Nature 1995;375:131–134.
Sheer CJ, Roberts JM . CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999;13:1501–1512.
Sheer CJ . Cancer cell cycles. Science 1996;274:1672–1677.
D’Agnano I, Valentini A, Fornari C, et al. Myc down-regulation induces apoptosis in M14 melanoma cells by increasing p27kip1 levels. Oncogene 2001;20:2814–2825.
Eymin B, Haugg M, Droin N, et al. p27KIP1 induces drug resistance by preventing apoptosis upstream to cytochrome c release and procaspase-3 activation in leukemic cells. Oncogene 1999;18:1411–1418.
Slingerland J, Pagano M . Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol 2000;183:10–17.
Kawana H, Tamaru J, Tanaka T, et al. Role of p27KIP1 and cyclin-dependent kinase 2 in the proliferation of non-small cell lung cancer. Am J Pathol 1998;153:505–513.
Gillett CE, Smith P, Peters G, et al. Cyclin-dependent kinase inhibitor p27Kip1 expression and interaction with other cyclin-associated proteins in mammary carcinoma. J Pathol 1999;187:200–206.
Catzavelos C, Bhattacharya N, Ung YC, et al. Decreased levels of the cell cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med 1997;3:227–230.
Loda M, Cukor B, Tam SW, et al. Increased proteasome-dependent degradation of the cyclin-dependent inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997;3:231–234.
Masciulo V, Sgambato A, Pacilio C, et al. Frequent loss of expression of the cyclin-dependent kinase inhibitor p27 in epithelial ovarian cancer. Cancer Res 1999;59:3790–3794.
Cheng JD, Werness BA, Babb JS, et al. Paradoxical correlations of cyclin-dependent kinase inhibitors p21waf1/cip1 and p27Kip1 in metastatic colorectal carcinoma. Clin Cancer Res 1999;5:1057–1062.
Anayama T, Furihata M, Ishikawa T, et al. Positive correlation between p27Kip1 expression and progression of human esophageal squamous cell carcinoma. Int J Cancer 1998;79:439–443.
Nycum LR, Smith LM, Farley JH, et al. The role of p27 in endometrial carcinoma. Gynecol Oncol 2001;81:242–246.
Watanabe J, Sato H, Kanai T, et al. Paradoxical expression of cell cycle inhibitor p27 in endometrioid adenocarcinoma of the uterine corpus-correlation with proliferation and clinicopathological parameters. Br J Cancer 2002;87:81–85.
Pagano M, Tam S, Theodoras A, et al. Role of the ubiquitin–proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 1995;269:682–685.
Tomoda K, Kubota Y, Kato JY . Degradation of the cyclin-dependent-kinase inhibitor p27kip1 is instigated by Jab1. Nature 398;1999:160–165.
Chopra S, de Mattos SF, Lam EW-F, et al. Jab1 co-activator of c-Jun is abrogated by serine 10-phosphorylated form of p27kip1. J Biol Chem 2002;277:32413–32416.
Claret FX, Hibi M, Dhut S, et al. A new group of conserved co-activators that increase the specificity of AP-1 transcription factors. Nature 1996;383:453–457.
Caballero OL, Resto V, Patturajan M . Interaction and colocalization of PGP9.5 with JAB1 and p27Kip1. Oncogene 2002;21:3003–3010.
Rassidakis GZ, Claret F-X, Lai R, et al. Expression of p27Kip1 and c-Jun activation binding protein 1 are inversely correlated in systemic anaplastic large cell lymphoma. Clin Cancer Res 2003;9:1121–1128.
Sui L, Dong Y, Ohno M, et al. Jab1 expression is associated with inverse expression of p27Kip1 and poor prognosis in epithelial ovarian tumors. Clin Cancer Res 2001;7:4130–4135.
Shen L, Tsuchida R, Miyauchi J, et al. Differentiation-associated expression and intracellular localization of cyclin-dependent kinase inhibitor p27kip1 and c-Jun co-activator JAB1 in neuroblastoma. Int J Oncol 2000;17:749–754.
Esteva FJ, Sahin AA, Rassidakis GZ, et al. Jun activation domain binding protein 1 (JAB1) expression is associated with low p27Kip1 levels in node-negative breast cancer. Clin Cancer Res 2003;9:5652–5659.
Morgan MB, Cowper SE . Expression of p-27 (kip1) in nevi and melanomas. Am J Dermpathol 1999;21:121–124.
Zhang H, Schneider J, Rosdahl I . Expression of p16, p27, p53, p73 and Nup88 proteins in matched primary and metastatic melanoma cells. Int J Oncol 2002;21:43–48.
Bales ES, Dietrich C, Bandyopadhyay D, et al. High levels of expression of p27 and cyclin E in invasive primary metastatic melanomas. J Invest Dermatol 1999;113:1039–1046.
Henriet P, Zhong Z-D, Brooks PC, et al. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27kip1. Proc Natl Acad Sci USA 2000;97:10026–10031.
Florenes VA, Maelandsmo GM, Kerbel RS, et al. Protein expression of the cell-cycle inhibitor p27kip1 in malignant melanoma: inverse correlation with disease-free survival. Am J Pathol 1998;153:305–312.
Hoos A, Urist MJ, Stijadinovic A, et al. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol 2001;158:1245–1251.
Hu F . Aging of melanocytes. J Invest Dermatol 1979;73:70–79.
Barbareschi M, van Tinteren H, Mauri FA, et al. P27(Kip1) expression in breast carcinomas: an immunohistochemical study on 512 patients with long-term follow-up. Int J Cancer 2000;89:236–241.
Volpi A, De Paola F, Nanni O, et al. Prognostic significance of biologic markers in node-negative breast cancer patients: a prospective study. Breast Cancer Res 2000;63:181–192.
Harper JW . Cyclin-dependent kinase inhibitors. In: Kastan MB (ed). Checkpoint Controls, Cancer, Cancer Surveys, Vol. 29. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 1997, pp 91–108.
Viglietto G, Motti ML, Bruni P . Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27kip1 by PKB/ Akt-mediated phosphorylation in breast cancer. Nat Med 2002;8:1136–1144.
Shing SP, Lipman J, Goldman H, et al. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinomas. Cancer Res 1998;58:1730–1735.
Thomas GV, Szigeti K, Murphy M, et al. Down regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am J Pathol 1998;153:681–687.
Shen L, Tsuchida R, Miyauchi J, et al. Differentiation-associated expression and intracellular localization of cyclin-dependent kinase inhibitor p27kip1 and c-Jun co-activator JAB1 in neuroblastoma. Int J Oncology 2000;17:749–754.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivan, D., Diwan, A., Esteva, F. et al. Expression of cell cycle inhibitor p27Kip1 and its inactivator Jab1 in melanocytic lesions. Mod Pathol 17, 811–818 (2004). https://doi.org/10.1038/modpathol.3800123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800123
Keywords
This article is cited by
-
Improved detection of gene-microbe interactions in the mouse skin microbiota using high-resolution QTL mapping of 16S rRNA transcripts
Microbiome (2017)
-
Jab1 promotes glioma cell proliferation by regulating Siah1/β-catenin pathway
Journal of Neuro-Oncology (2017)
-
Adhesion to fibronectin induces p27Kip1 nuclear accumulation through down-regulation of Jab1 and contributes to cell adhesion-mediated drug resistance (CAM-DR) in RPMI 8,226 cells
Molecular and Cellular Biochemistry (2014)
-
Immunohistochemical Expression of p16, p21, p27 and Cyclin D1 in Oral Nevi and Melanoma
Head and Neck Pathology (2012)
-
Molecular targeting of CSN5 in human hepatocellular carcinoma: a mechanism of therapeutic response
Oncogene (2011)