Abstract
To determine the role of cytokines in acute myocarditis, we examined expressional patterns of cardiotrophin-1 (CT-1), TNF-α, and IL-1α in a murine model of acute myocarditis. Ten-day-old Institute of Cancer Research mice were injected with Coxsackievirus B3 and killed on Days 1, 2, 3, 4, 5, 7, 10, 14, and 28 of injection. TNF-α and IL-1α expressions were investigated on histological sections from each heart, and mRNA expression of TNF-α, IL-1α, and CT-1 in the heart was examined by reverse transcription-polymerase chain reaction and RNase protection assay. To determine myocardial regeneration, cardiomyocytic DNA synthesis was investigated using bromodeoxyuridine on Days 3, 5, 7, and 10, and the labeling index was calculated in each heart. Age-matched uninfected mice were used as controls. TNFα and IL-1α expression was first detected in the cardiomyocytes on Day 3 and reached the maximum level on Day 7, when inflammatory changes were most prominent. Although an increased expression of TNFα and IL-1α mRNA was also detected on Day 3, CT-1 mRNA expression was distinctly augmented on Day 2. The labeling indices in the hearts with myocarditis were significantly higher than in those of the controls in all of the time points examined. CT-1 expression preceded TNF-α and IL-1α expressions and active DNA synthesis in a murine model of acute myocarditis. All CVB3-infected mice with anti-glycoprotein-130 antibody treatment died within 6 days. CT-1 may exert a protective role by modulating cytokine production and by inducing cardiomyocytic proliferation in CVB3-infected murine hearts.
Similar content being viewed by others
Introduction
Viral myocarditis results from a viral infection that induces myocardial necrosis and triggers a series of immune responses to eliminate the viral agent. Although several studies have examined the crucial roles of cytokines and the induction of apoptosis in the myocardium, the pathogenic mechanisms of myocarditis remain unclear (Bowles and Towbin, 1998; Bryant et al, 1998; Henke et al, 1992; Hober et al, 1996; Huber et al, 1996, 1998; Kishimoto et al, 1994; Matsumori, 1997; Okura et al, 1998; Satoh et al, 1996; Seko et al, 1997, 1998; Shioi et al, 1996; Torre-Amione et al, 1995). Cytokine production and apoptosis may contribute to myocardial cell loss, which results in compensatory myocardial hypertrophy, fibrosis, scarring, and cardiac dilation (Suddaby, 1996). However, it is clinically known that most patients recover spontaneously, and, in fact, histological studies of autopsied specimens from children who died of acute myocarditis revealed increased myocardial nuclear divisions (Liu et al, 1996; MacMahon, 1937). Therefore, with the exception of the compensatory response, some regeneration or restoration of heart muscle cells must occur as a supportive response in acute myocarditis.
Recent studies show that cardiotrophin-1 (CT-1), a potent cardiac survival factor and a member of the IL-6 family of cytokines, inhibits cytokine-induced apoptosis in cardiac myocytes (Benigni et al, 1996; Pulkki, 1997; Sheng et al, 1997). Although these studies suggest a protective role of CT-1 in cytokine-induced myocardial damage, few precise studies describing expression of CT-1 in myocarditis have been reported (Chandrasekar et al, 1998).
To determine the role of cytokines in pathologic mechanisms of acute viral myocarditis, we examined the mRNA expression and the immunohistochemical expression patterns of TNF-α and IL-1α in a murine model of Coxsackievirus B3 (CVB3)-induced acute myocarditis. We also examined the time course of CT-1 mRNA expression in the heart of this animal model using RNase protection assay. Furthermore, to evaluate the pathologic role of CT-1 in myocardial damage, we administered an anti-glycoprotein (gp) 130 antibody to CVB3-infected mice. In addition, to assess the occurrence of myocardial regeneration or proliferation, we investigated cardiomyocytic DNA synthesis in acute myocarditis using the bromodeoxyuridine (BrdU) flash-labeling method.
Results
Animal Survival
Only 18 of 100 mice injected with CVB3 died between Days 3 and 7 of injection, and the survival rate at 7days was 82%. Histologic examinations of some of their hearts showed massive myocardial degeneration and widespread myocardial cell loss; however, the findings might have been caused by postmortem changes. The other 82 mice survived, although they appeared slightly ill during the first week after CVB3 injection (Fig 1).
Histologic Findings from the CVB3-Infected Hearts
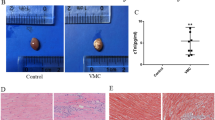
Myocardial degeneration and inflammatory cellular infiltration were first detected around vessels on Day 4 of CVB3 injection (Fig. 2A) and were most prominent on Day 7 (Fig. 2, B and C). Myocardial cell loss and proliferative fibroblasts were also observed on Day 7. These pathologic findings, typical of acute myocarditis, were observed in all hearts removed on Days 5, 7, and 10, although there was some variation among the hearts. The inflammatory cellular infiltration was persistent, although it decreased on Days 14 and 28 of injection (Fig. 2D). As the scarring and fibrosis spread, the numbers of inflammatory cells increased in the hearts removed on Days 14 and 28 of CVB3 injection.
Histologic findings of murine hearts on Days 4 (A), 7 (B and C), and 28 (D) after CVB3 injection. A, Although inflammatory cellular infiltrations are dominantly observed around vessels (arrowheads), no myocardial degeneration is seen at this stage. B, Myocardial degeneration and infiltration of inflammatory cells are prominent all over the histological section. C, Higher magnification of B. D, Infiltrating inflammatory cells are decreased, and scarring and fibrosis were observed to replace the degenerated myocardium. Bars = 20 μm.
Expression of the Cytokines in the CVB3-Infected Hearts
The TNF-α expression was first detected on sections from hearts removed on Day 3 of CVB3 injection, when no infiltration of inflammatory cells was detected (Fig. 3A). The immunodistribution of TNF-α was observed to spread wider thereafter (Fig. 3B). The most widespread TNF-α expression was detected on Day 7, and TNF-α was distributed all over the heart (Fig. 3C). The expression of TNF-α was detected on the surface of viable cardiac myocytes rather than around the infiltrating cells on Days 3, 5, and 7. Reduced TNF-α expression was only detected in the interstitial tissue of Day 28 hearts along with heavy fibrosis (Fig. 3D). Expression of IL-1α in CVB3-infected hearts was almost the same, although reduced, as was that of TNF-α (Fig. 3E-H). IL-1α was also predominantly detected in the cardiac myocytes. An increase in TNF-α and IL-1α mRNA expressions was detected on Day 3 of CVB3 injection (Fig 4). CT-1 mRNA expression was distinctly augmented in the hearts with myocarditis removed on Day 2 of CVB3 injection, as shown in Fig 5.
Immunohistochemical study of murine myocarditis for TNF-α (A to D) and IL-1α (E to H). A, The TNF-α expression was first observed in the myocardial cells as early as Day 3 after CVB3 injection. B, Note wide spread of TNF-α expression in the myocardial cells on Day 4. C, Prominent TNF-α expression was observed around the degenerative myocardial cells on Day 7. D, TNF-α expression was only observed in the infiltrating cells around the interstitial tissue on Day 28. E to H, IL-1α expression observed on Days 3 (E), 4 (F), 7 (G), and 28 (H) after CVB3 injection. Bars = 20 μm.
Expressions of TNF-α and IL-1α mRNAs in murine myocarditis. Total RNA was prepared from the hearts obtained on Days 0, 1, 2, 3, 4, and 5 after CVB3 injection; mRNA expressions were examined by RT-PCR. Glycer-aldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. A, Equal volumes of the amplified products were loaded onto 2%-agarose gel. B, The relative intensity of the bands for TNF-α and IL-1α mRNA obtained by densitometry was divided by the intensity of the bands for the internal marker, GAPDH. Values are means ± se from five samples. *† p < 0.05 versus Day 3.
Expression of cardiotrophin (CT)-1 mRNA in murine myocarditis. Total RNA was prepared from the hearts obtained on Days 0, 1, 2, 3, and 7 after CVB3 injection. CT-1 mRNA expression was examined by the RNase protection assay. Mouse β-actin probe was used as an internal control. The results were from three independent experiments.
Labeling Index with BrdU
The labeling index (LI) with BrdU of cardiomyocytes is shown in Fig. 6. The LI in the myocarditis group was significantly higher than that of controls (p <0.05) at all stages examined. The highest LI was 3.0 ± 0.37% on Day 10 of injection. There was no difference in LI among three regions of the heart. BrdU immunoreactivity was not only detected on the nuclei of cardiomyocytes but on those of fibroblasts, endothelial, and infiltrating cells (Fig. 7).
Effect of Anti-gp130 Antibody on the Survival of CVB3-Infected Mice
All CVB3-infected mice with an anti-mouse gp130-antibody (RX435) treatment (n = 4) died before Day 6 of injection (Fig 1), although the mouse treated with RX435 without CVB3 infection survived. Histologic sections from the mouse treated with RX435 alone revealed no pathological changes.
Discussion
We demonstrated the histopathologic changes and expression of cytokines in CVB3-infected mouse hearts. Because myocarditis is more severe in young subjects with acute heart failure and more insidious in adults with chronic dilated cardiomyopathy (Liu et al, 1996), we used 10-day-old mice in our study. Because all mice injected with CVB3 showed histologic myocarditis with some variation, including the 18% that died, the age group of mice used in this study appeared adequate to investigate the pathogenic mechanism of acute myocarditis.
This study revealed a few important findings suggesting the crucial roles of cytokines in acute myocarditis. The first was the distinct detection of CT-1 mRNA expression in acute myocarditis, which preceded TNF-α and IL-1α mRNA expressions. This is the first study to reveal CT-1 mRNA expression in acute myocarditis. The second was the immunohistochemical detection of TNF-α and IL-1α expressions on the surface of cardiomyocytes before the occurrence of myocardial degeneration or inflammatory cellular infiltration. The significance of our observation is that cardiomyocytes infected with CVB3 in vivo produce TNF-α and IL-1α, which are proinflammatory cytokines and thought to be produced by inflammatory cells, including lymphocytes, monocytes, and/or macrophages (Lane et al, 1992, 1993). This observation indicates that TNF-α and IL-1α released from infected cardiomyocytes induce infiltration of inflammatory cells, including natural killer cells, helper T cells, and cytotoxic T cells, resulting in myocardial degeneration. In addition, as Bryant et al (1998) reported, production of TNF-α by cardiomyocytes causes severe myocardial damage in transgenic mice, including a globular dilated heart, myocardial apoptosis, and transmural myocarditis; it may also induce myocardial degeneration in an autocrine and/or paracrine fashion.
Our results correspond to those of previous studies showing TNF-α or TNF-α mRNA expression in myocardial cells infected with CVB3 in vivo and in vitro almost throughout the early phase of infection (Matsumori et al, 1994; Seko et al, 1997). However, Lane et al (1992) reported that the serum concentrations of TNF-α and IL-1α in a murine model of autoimmune myocarditis increased 2 weeks after CVB3 injection, which was associated with infiltration of TNF-α or IL-1α immunopositive T cells and macrophages. Because their observation differs from the results of our study, the acute myocarditis in our mice appears to be caused by mechanisms other than autoimmunity. CVB3 infection and subsequent cytokine production from myocardial cells appear to play a direct causative role in the pathogenesis of acute myocarditis, although the latter might be a part of the self-protective mechanism to eliminate infected cells. Recent studies have suggested that CT-1 inhibits cardiac myocyte apoptosis in vitro (Pulkki, 1997; Sheng et al, 1997) by activating mitogen-activated protein kinase-dependent pathways. Benigni et al (1996) reported that CT-1 co-treated with lipopolysaccharide, an inducer of cytokine, markedly inhibited TNF-α production in serum and in the heart. These studies suggest a protective role of CT-1 in cytokine-induced myocardial damage. Because CT-1 transduces its signals via a gp130-dependent signaling pathway (Wollert et al, 1996; Wollert and Chien, 1997), CT-1 signaling can be inhibited by using an anti-gp130 antibody. Although only four mice were examined in our study, all of the mice infected with CVB3 with anti-gp130-antibody treatment died in the early phase of acute myocarditis. This result strongly supports the protective role of IL-6–related cytokines, especially CT-1, in acute myocarditis. Therefore, the enhanced expression of CT-1 mRNA, preceding TNF-α and IL-1α mRNA up-expression, might promote cardiac myocyte survival against viral infection and apoptosis by inhibiting the production of pro-inflammatory cytokines.
TNF-α expression was not detected in cardiomyocytes but in persistently infiltrating cells in the interstitial tissue on Day 28. Because this TNF-α expression was only detected in hearts with severe myocardial cell loss, fibrosis, and persistent inflammatory cells, subsequent production of TNF-α might be related to chronic myocarditis or dilated cardiomyopathy (Hober et al, 1996; Shioi et al, 1996).
A significant increase of DNA-synthesizing cardiomyocytic nuclei was detected in mice hearts with CVB3-induced myocarditis. Although it is generally thought that regeneration seldom occurs in the heart, several studies on DNA synthesis of cardiomyocytes have been reported in adult humans and animals under certain strong stimuli or overload (Linzbach, 1976; Nag and Cheng, 1981; Vracko and Thorming, 1985). Nakagawa et al (1988), studying proliferative activity of cardiomyocytes in ICR mice aged 1 to 200 days, reported that cardiomyocytic DNA synthesis was most active in the first 14 days and retained thereafter, although at low levels. The heart muscle cells examined in this study maintained DNA synthetic activity. The higher LI in the myocarditis group than that of the control group indicates that DNA synthesis in cardiomyocytic nuclei is activated in acute myocarditis. (Chandrasekar et al, 1988) investigated CT-1 and CT-1 mRNA expressions in Lewis rat myocardial cells during acute Chagasic carditis and reported a marked elevation on Day 15 of cruzi trypomastogotes inoculation. Although they suggest that the overexpression of CT-1 and CT-1 mRNA is a mechanism for myocyte protection and for compensatory hypertrophy (Chandrasekar et al, 1998), our study showed that the LI on Day 3 was already significantly higher than that of the control, when severe myocardial cell loss had not been evident. Therefore, it is difficult to accept that the DNA synthesis in cardiomyocytic nuclei was activated only for compensatory hypertrophy. Because the mice cardiomyocytic DNA synthesis results in binucleation (Nakagawa et al, 1988), the early expression of CT-1 mRNA might induce extensive binucleation of heart muscle cells via DNA synthesis as a survival response to myocardial damage. The most active DNA synthesis in myocardial nuclei on Day 10 after CVB3 injection can be considered to lead to the compensatory hypertrophy.
Conclusions
We concluded that cardiac myocytes infected with CVB3 express cytokines, including CT-1, TNFα, and IL-1α in autocrine and/or paracrine fashion, which induce pathologic responses in acute myocarditis. Early expression of CT-1 may play a protective role by inhibiting TNF-α and IL-1α expressions and by inducing DNA synthesis in cardiac myocytes.
Methods and Materials
Animals
Male Institute of Cancer Research mice, obtained from Charles River, Shizuaka, Japan and bred in the animal institute of Shiga University of Medical Science (Shiga, Japan) were used for the study. Experiments were carried out in triplicate using five mice for each treatment. The experiments were conducted with strict adherence to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and the standards of the Institutional Animal Care and Use Committee, Shiga University of Medical Science.
Materials
The following chemicals were used for the study: minimal essential medium (GIBCO BRL, Gaithersburg, Maryland), fetal bovine serum (GIBCO BRL), BrdU (Sigma, St. Louis, Missouri), anti-rat BrdU antibody (BioSys, Copenhagen, Denmark), anti-mouse TNF-α polyclonal antibody (Endogen, Boston, Massachusetts), anti-human IL-1α polyclonal antibody (Endogen), avidin-biotin-horseradish peroxidase complex (Vectastain ABC kit, Vector Laboratories, Burlingame, California), 3,3′-diaminobenzidine (Vectastain ABC kit), proteinase K (Dako, Carpinteria, California), labeled streptoavidin-biotin (Dako), and 3-amino-9-ethylcarbazole (Dako).
Virus
CVB3 Nancy strain provided by Dr. Tetsuo Yoneyama (Department of Virology II, National Institute of Infectious Diseases, Tokyo, Japan) was used to produce the experimental infection after its passage in tissue culture, as previously described (Rubin et al, 1998). In brief, stocks of each strain were prepared from virus passaged twice on HEp-2 cells in minimal essential medium with 5% fetal bovine serum at 37° C.
Production of CVB3-Induced Acute Myocarditis
Each mouse was injected intraperitoneally with 0.02 ml·g−1 weight of minimal essential medium of stock virus containing 2 × 104 median tissue culture infective dose (TCD50). A time-course experiment was set up to delineate the progression of myocarditis on the histological sections. Sixty 10-day-old mice received CVB3 on Day 0 of injection. Five of the 60 mice were killed under deep anesthesia with ether on Days 1, 2, 3, 4, 5, 7, 10, 14, and 28 after injection. A single intraperitoneal injection of 50 μg·g−1 weight of BrdU was given to each mouse 1 hour before they were killed on Days 3, 5, 7, and 10. Age-matched uninfected mice were used as controls.
Anti-gp130 Blocking Antibody Injection
An anti-mouse gp130 antibody (RX435) was used to inhibit CT-1 signaling. Four mice infected with CVB3 and one age-matched uninfected mouse were injected subcutaneously with 40 μg·g−1 weight of RX435 on Day 1 of CVB3 injection.
Immunohistochemical Studies
After the mice had been killed, the hearts were immediately removed and fixed in 4% paraformaldehyde for 24 hours. They were embedded in paraffin and serial 5-μm sections were made. Every 10th section was stained with hematoxylin eosin for histopathologic evaluation of myocarditis. Subsequent sections were subjected to immunohistochemical staining with anti-mouse TNF-α polyclonal antibody, anti-human IL-1α polyclonal antibody, and anti-rat BrdU antibody. Deparaffinized and hydrated sections were incubated for 30 minutes in 0.3% H2O2 in methanol to quench endogenous peroxidase activity, rinsed for 20 minutes with phosphate-buffered saline, then incubated for 30 minutes with diluted normal species-blocking serum. Sections were incubated overnight with anti-TNF-α antibody (diluted 1:100) or with anti-IL–1α antibody (diluted 1:100) in humidified air. Immune complexes were subsequently detected with biotinylated goat antibodies to mouse immunoglobulin G for TNF-α and to human immunoglobulin G for IL-1α, followed by avidin-biotin-horseradish peroxidase, and incubated for 5 minutes with 0.1% 3,3′-diaminobenzidine and 0.02% hydrogen peroxide. Background staining, routinely negligible, was controlled with normal serum replacing the primary antiserum.
RNase Protection Assay
Forty 10-day-old mice received CVB3 on Day 0 of injection. Five mice were killed under deep anesthesia with ether on Days 0, 1, 2, 3, and 7 of injection. Age-matched uninfected mice were used as controls. The hearts were immediately removed and rinsed with ice-cold phosphate-buffered saline, and total cellular RNA was isolated by the acid-guanidinium thiocyanate-phenol-chloroform method (Chomczynski and Sacchi, 1987). A CT-1 cDNA was obtained by reverse transcription (RT)-polymerase chain reaction (PCR) methods using specific oligonucleotides primers (sense, 5′-GAGACAGTGCTGGCCGGCGCTG-3′; anti-sense, 5′-AGAGGAGAGCAGAAGAGAGAGA-3′) synthesized based on the nucleotide sequence of mouse CT-1 cDNA (Pennica et al, 1995). Resulting products of 345 bp in size were subcloned into pGEM (Promega, Madison, Wisconsin) and linealized by digestion with NcoI. The subcloned DNA was sequenced using an automatic DNA sequencer (ABI PRISM 310; Applied Biosystems, Foster City, California) to confirm its identity. The sequence of the PCR product was identical to the corresponding sequence of mouse CT-1 cDNA. RNase protection assays were performed with the Hybspeed RPA kit (Ambion, Inc., Austin, Texas) according to the manufacturer’s instructions. In brief, the [α-32P] CTP-labeled RNA probe (345 bp) was generated according to the technical manual for the Maxi script kit (Ambion, Inc.). The probe (1–2 × 105 cpm) was hybridized with 5–10 μg of total RNA at 68° C for 20 minutes in the hybridization buffer. After digestion with RNase, the protected RNA was precipitated with ethanol and loaded onto a 5% polyacrylamide gel. A mouse β-actin probe (250 bp) was used as an internal control. Autoradiography was performed at −80° C for 6 to 96 hours.
Semiquantitative RT-PCR
Forty 10-day-old mice received CVB3 on Day 0 of injection. Five mice were killed under deep anesthesia with ether on Days 0, 1, 2, 3, 4, and 5 of injection. Age-matched uninfected mice were used as controls. Total RNA (0.1 μg), isolated by the same method as used in RNase protection assay, was subjected to first-strand synthesis by using the oligo (dT) (Amersham Pharmacia, Tokyo, Japan) and Moloney murine leukemia virus reverse transcriptase (GIBCO/BRL) at 37° C for 1 hour (Fujio et al, 1997). The first-strand DNA was amplified by PCR with the following sets of primers: TNF-α (Ikegami et al, 1995), 5′-GCCTCTTCTCATTCCTGCTT-3′ and 5′-ACTTGG-TGGTTTGCTACGAC-3′; IL-1α (Gene Bank Accession No. E04743), 5′-CAAATCTCACAGCAGCACA-3′ and 5′-CAGGTTATCATCATCATCCC-3′. PCR was performed for 30 cycles as follows: initial denaturation at 94° C for 5 minutes, further denaturation at 94° C for 60 seconds, annealing for 90 seconds with different temperatures for each primer (TNF-α, 56° C and IL-1α, 52° C), a polymerization step at 72° C for 60 seconds, and a final extension at 72° C for 10 minutes. Specific primers for glycer-aldehyde-3-phosphate dehydrogenase (GAPDH) (Tokunaga et al, 1987) (5′-GCCAAAAGGGTCATCATCTCTG-3′ and 5′-CAT- GCCAGTGAGCTTCCCGT-3′) were also used to measure quality and quantity of RNA in each sample. PCR-amplified cDNAs were directly sequenced on an ABI Prism 310 Genetic Analyzer (Applied Biosystems) by dye-terminator chemistry. Equal volumes of the PCR products were loaded onto a 2%-agarose gel and stained with ethidium bromide. The intensity of the bands was analyzed by densitometry (AIC Epi-Light UV FA1100, AISIN COSMOS R&D, Tokyo, Japan).
Calculation of LI with BrdU
The sections stained with anti-BrdU antibody were used to calculate LI with BrdU of cardiomyocytes. Immune complexes were detected with biotinylated goat antibodies to rat immunoglobulin G, followed by labeled streptoavidin-biotin, and incubated for 5 minutes with 3% 3-amino-9-ethylcarbazole. LI was calculated in each heart and expressed as a ratio of labeled nuclei in a total of 10,000 cardiomyocytic nuclei in three different parts of the heart as follows: the left ventricular free wall, the interventricular septum, and the right ventricular free wall.
Statistics
LI with BrdU of cardiomyocytes from infected and uninfected mice were compared by t tests. A p value = 0.05 was considered statistically significant.
Accession codes
References
Benigni F, Sacco S, Pennica D, and Ghezzi P (1996). Cardiotrophin-1 inhibits tumor necrosis factor production in the heart and serum of lipopolysaccharide-treated mice and in vitro in mouse blood cells. Am J Pathol 149: 1847–1850.
Bowles NE, and Towbin JA (1998). Molecular aspects of myocarditis. Curr Opin Cardiol 13: 179–184.
Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, and Giroir B (1998). Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation 97: 1375–1381.
Chandrasekar B, Melby PC, Pennica D, and Freeman GL (1998). Overexpression of cardiotrophin-1 and gp130 during experimental acute Chagasic cardiomyopathy. Immunol Lett 61: 89–95.
Chomczynski P and Sacchi N (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159.
Fujio Y, Kunisada K, Hirota H, Yamauchi-Takihara K, and Kishimoto T (1997). Signals through gp130 upregulate bcl-x gene expression via STAT1-binding cis-element in cardiac myocytes. J Clin Invest 99: 2898–2905.
Henke A, Spengler HP, Stelzner A, Nain M, and Gemsa D (1992). Lipopolysaccharide suppresses cytokine release from Coxsackie virus-infected human monocytes. Res Immunol 143: 65–70.
Hober D, Andreoletti L, Shen L, Copin MC, Desmidt A, and Wattre P (1996). Coxsackievirus B3-induced chronic myocarditis in mouse: Use of whole blood culture to study the activation of TNF alpha-producing cells. Microbiol Immunol 40: 837–845.
Huber SA, Gauntt CJ, and Sakkinen P (1998). Enteroviruses and myocarditis: Viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv Virus Res 51: 35–80.
Huber SA, Mortensen A, and Moulton G (1996). Modulation of cytokine expression by CD4+ T cells during coxsackievirus B3 infections of BALB/c mice initiated by cells expressing the gamma delta + T-cell receptor. J Virol 70: 3039–3044.
Ikegami H, Makino S, Yamato E, Kawaguchi Y, Ueda H, Sakamoto T, Takekawa K, and Ogihara T (1995). Identification of a new susceptibility locus for insulin-dependent diabetes mellitus by ancestral haplotype congenic mapping. J Clin Invest 96: 1936–1942.
Kishimoto C, Kuroki Y, Hiraoka Y, Ochiai H, Kurokawa M, and Sasayama S (1994). Cytokine and murine Coxsackievirus B3 myocarditis. Interleukin-2 suppressed myocarditis in the acute stage but enhanced the condition in the subsequent stage. Circulation 89: 2836–2842.
Lane JR, Neumann DA, Lafond-Walker A, Herskowitz A, and Rose NR (1992). Interleukin 1 or tumor necrosis factor can promote Coxsackie B3-induced myocarditis in resistant B10. A mice. J Exp Med 175: 1123–1129.
Lane JR, Neumann DA, Lafond-Walker A, Herskowitz A, and Rose NR (1993). Role of IL-1 and tumor necrosis factor in Coxsackievirus-induced autoimmune myocarditis. J Immunol 151: 1682–1690.
Linzbach AJ (1976). Hypertrophy, hyperplasia and structural dilatation of the human heart. Adv Cardiol 18: 1–4.
Liu P, Martino T, Opavsky MA, and Penninger J (1996). Viral myocarditis: Balance between viral infection and immune response. Can J Cardiol 12: 935–943.
MacMahon HE (1937). Hyperplasia and regeneration of the myocardium in infants and in children. Am J Pathol 8: 845–852.
Matsumori A (1997). The use of cytokine inhibitors. A new therapeutic insight into heart failure. Int J Cardiol 62 Suppl 1: S3–12.
Matsumori A, Yamada T, Suzuki H, Matoba Y, and Sasayama S (1994). Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J 72: 561–566.
Nag AC and Cheng M (1981). Adult mammalian cardiac muscle cells in culture. Tissue Cell 13: 515–523.
Nakagawa M, Hamaoka K, Hattori T, and Sawada T (1988). Postnatal DNA synthesis in hearts of mice: Autoradiographic and cytofluorometric investigations. Cardiovasc Res 22: 575–583.
Okura Y, Takeda K, Honda S, Hanawa H, Watanabe H, Kodama M, Izumi T, Aizawa Y, Seki S, and Abo T (1998). Recombinant murine interleukin-12 facilitates induction of cardiac myosin-specific type 1 helper T cells in rats. Circ Res 82: 1035–1042.
Pennica D, King KL, Shaw KJ, Luis E, Rullamas J, Luoh SM, Darbonne WC, Knutzon DS, Yen R, Chien KR, Baker JB, and Wood WI (1995). Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci USA 92: 1142–1146.
Pulkki KJ (1997). Cytokines and cardiomyocyte death. Ann Med 29: 339–343.
Rubin SA, Pletnikov M, and Carbone KM (1998). Comparison of the neurovirulence of a vaccine and a wild-type mumps virus strain in the developing rat brain. J Virol 72: 8037–8042.
Satoh M, Tamura G, Segawa I, Tashiro A, Hiramori K, and Satodate R (1996). Expression of cytokine genes and presence of enteroviral genomic RNA in endomyocardial biopsy tissues of myocarditis and dilated cardiomyopathy. Virchows Arch 427: 503–509.
Seko Y, Takahashi N, Azuma M, Yagita H, Okumura K, and Yazaki Y (1998). Expression of costimulatory molecule CD40 in murine heart with acute myocarditis and reduction of inflammation by treatment with anti-CD40L/B7–1 monoclonal antibodies. Circ Res 83: 463–469.
Seko Y, Takahashi N, Yagita H, Okumura K, and Yazaki Y (1997). Expression of cytokine mRNAs in murine hearts with acute myocarditis caused by Coxsackievirus B3. J Pathol 183: 105–108.
Sheng Z, Knowlton K, Chen J, Hoshijima M, Brown JH, and Chien KR (1997). Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem 272: 5783–5791.
Shioi T, Matsumori A, and Sasayama S (1996). Persistent expression of cytokine in the chronic stage of viral myocarditis in mice. Circulation 94: 2930–2937.
Suddaby EC (1996). Viral myocarditis in children. Crit Care Nurse 16: 73–82.
Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, and Sakiyama S (1987). Enhanced expression of a glyceraldehyde-3-phodsphate dehydrogenase gene in human lung cancers. Cancer Res 47: 5616–5619.
Torre-Amione G, Kapadia S, Lee J, Bies RD, Lebovitz R, and Mann DL (1995). Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation 92: 1487–1493.
Vracko R and Thorming D (1985). Freeze-thaw injury of rat heart across an intact diaphragma: A new model for the study of the response of myocardium to injury. Cardiovasc Res 19: 76–84.
Wollert KC and Chien KR (1997). Cardiotrophin-1 and the role of gp130-dependent signaling pathways in cardiac growth and development. J Mol Med 75: 492–501.
Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, Vernallis AB, Heath JK, Pennica D, Wood WI, and Chien KR (1996). Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series via gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem 271: 9535–9545.
Acknowledgements
We thank Dr. Tetsuo Yoneyama for providing the CVB3 Nancy strain and Dr. Tomoyuki Takano for his advice on propagating the virus. We also thank Drs. Masaki Suzaki, Yoshihiro Maruo, Tsutomu Narita, and Noriko Watanabe for expert technical assistance in RT-PCR.
Supported by a Grant-in-Aid for Scientific Research (C) from The Japanese Ministry of Education, Science, Sports and Culture, Numbers: 05670671, 07670857, 09670800, and 09670717, and by Grants from the Ministry of Health and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okuno, M., Nakagawa, M., Shimada, M. et al. Expressional Patterns of Cytokines in a Murine Model of Acute Myocarditis: Early Expression of Cardiotrophin-1. Lab Invest 80, 433–440 (2000). https://doi.org/10.1038/labinvest.3780048
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3780048