Abstract
Malignant peripheral nerve sheath tumor (MPNST) is rare, highly aggressive, resistant to radiochemotherapy, and associated with poor prognosis. Basic research to develop new treatment regimes is critically needed. This study was designed to identify motogenic factor(s) involved in MPNST cell invasion and inhibitor(s) of such invasive activity. We profiled the invasion-inducing activities of eight motogenic growth factors on two human MPNST cell lines, FU-SFT8611 and 9817, using in vitro Matrigel invasion assays. Platelet-derived growth factor-BB (PDGF-BB) was identified as the most effective MPNST cell invasion-inducing factor. Epidermal growth factor (EGF) and hepatocyte growth factor (HGF) also stimulated invasion in one MPNST cell line. Expressions of PDGF-BB and EGF receptors (PDGFR-β and EGFR) mRNAs were detected more frequently and their proteins were expressed at higher levels in MPNST tissues than benign peripheral nerve sheath tumors (schwannomas and neurofibromas). In both MPNST cell lines, PDGF-BB induced tyrosine phosphorylation of PDGFR-β but not of PDGFR-α, and specific PDGFR-β inhibition by small interfering RNA to the receptor inhibited PDGF-BB-stimulated MPNST cell invasion, suggesting the predominant role of PDGFR-β. Inhibition of PDGFR-β phosphorylation by pretreatment with herbimycin A and imatinib mesylate effectively suppressed basement membrane invasion and cell growth in vitro. No mutations were present in exons 12 and 18 of PDGFR-β in both MPNST cell lines and 10 human MPNST tissues examined. Our results indicated that PDGF-BB enhanced the invasive activity of MPNST cells through PDGFR phosphorylation and that imatinib inhibited such activity. The results provide the ground for further assessment of the therapeutic potential of imatinib in suppressing the invasion and growth of MPNST.
Similar content being viewed by others
Main
Malignant peripheral nerve sheath tumor (MPNST) is an uncommon and highly progressive soft tissue tumor arising from either neurofibromatosis type 1 (NF1) or de novo from peripheral nerves, accounting for about 5% of malignant tumors of soft tissue.1, 2, 3 The overall 5- and 10-year survival rate were 34 and 22%, respectively.4 The rates of successful treatment of MPNST are generally low, and the available literature provides only limited guidance regarding the management of this disease.5, 6 Surgical removal is presently the sole effective treatment and the goal of surgery is complete resection with negative margin. No data are available to prove that adjuvant radiotherapy or chemotherapy is of any value in improvement of overall survival of MPNST patients. However, while radiotherapy dose not improve the long-term survival of patients with MPNSTs, it helps control tumor growth locally.7 There are currently only a few treatment options available for patients with advanced and multiple recurrent MPNST.
Overexpression of growth factors and/or their receptors is believed to play an important role in cellular transformation. Rat Schwann cells respond to some mitogens in vitro, such as transforming growth factor-β (TGF-β), hepatocyte growth factor (HGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF),8, 9, 10 and human neurofibromatosis tissues overexpress HGF and basic FGF (bFGF).11, 12 However, their role in the pathogenesis of MPNST remains unclear. It was reported that PDGF-BB is mitogenic for two human MPNST cell lines, but not for a Schwann cell line derived from a schwannoma.13 Significantly high expression levels of both PDGF receptor (PDGFR)-α and -β were found in the two MPNST cell lines compared to non-NF1 Schwann cell lines. Furthermore, the level of tyrosyl-phosphorylated PDGFR-β was strongly increased upon stimulation by PDGF-BB. PDGF-AA was only a weak mitogen for MPNST cells compared with PDGF-BB.13 An immunohistochemical study of MPNST arising within neurofibroma associated with NF1, demonstrated more frequent positive reaction with PDGF in the MPNST areas than in the neurofibroma areas in two of eight cases.14 However, positive reactions with PDGFR-β were seen in only one case. Another report showed that PDGFR-β expression levels were similar in normal human Schwann cells and NF1 MPNST cell lines, as examined by immunoblotting, but that increased intracellular calcium (Ca2+) levels in response to PDGF-BB were observed only in the NF1 MPNST cell lines. Therefore, PDGF-BB induced higher levels of proliferation in MPNST cell lines than in normal human adult Schwann cells.15 The above studies were concerned with the mitogenic activity of growth factors, and few papers have investigated motogenic or invasion-enhancing activity of growth factors in MPNSTs; for example, HGF-promoted MPNST cell invasion through an HGF/c-Met autocrine loop.16 Acquisition of invasive properties also plays an important role in tumor progression. The present study was designed to identify motogenic factor(s) involved in MPNST cell invasion and find effective inhibitor(s) of the invasive activity.
Materials and methods
Growth Factors and Inhibitors
PDGF-BB, PDGF-AA, epidermal growth factor (EGF), bFGF, acidic FGF (aFGF), insulin-like growth factor (IGF), HGF and TGF-β1 were purchased from Peprotech Inc. (Rocky Hill, NJ, USA). Herbimycin A was obtained from Calbiochem (La Jolla, CA, USA), and imatinib mesylate (Gleevec/STI571) was a generous gift from Novartis Pharma AG (Basel, Switzerland).
Cell Culture
FU-SFT8611 and FU-SFT9817 cell lines were established from a 40-year-old Japanese man and a 61-year-old Japanese woman, both free of NF1, respectively, as described previously.17 These cell lines exhibited complex karyotypes lacking a common characteristic pattern, and were tumorigenic in nude mice, with the resultant tumors showing an immunohistochemical phenotype similar to the original tumors. Comparative genomic hybridization analysis revealed that chromosomal imbalances were very similar between the original tumors and the established cell lines, but no consistent imbalances between the two cell lines as reported so far. Both MPNST cell lines were maintained in growth medium (GM), a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Rockville, MD, USA) and Ham's F12 (Nissui Seiyaku, Tokyo, Japan), supplemented with 10% fetal calf serum (FCS), L-glutamine (746 μg/ml), sodium bicarbonate (0.2%), streptomycin (90 μg/ml) and penicillin G (90 μg/ml), pH 7.35.
In Vitro Invasion Assays
The invasion assay was performed as described previously using modified fluoroblock invasion assay (BD BioCoat Tumor Invasion system, BD Biosciences, Franklin Lakes, NJ, USA).18 Briefly, MPNST cells grown to preconfluence were labeled with 10 ng/ml Di1 (Invitrogen, Carlsbad, CA, USA) in GM overnight, and the labeled cells (3.2 × 105/ml) were placed in the upper compartment of a fluoroblock 24-multiwell insert plate, which was separated from the lower compartment by a Matrigel (25 μg)-coated fluoroblock membrane with 8.0-μm pore size. In both compartments, the incubation medium was serum-free DMEM/F12, supplemented with 0.1% bovine serum albumin (BSA). Growth factors were added to the lower compartment, and 10% FCS added to the lower compartment served as a positive control. After incubation for 24–72 h at 37°C in a 5% CO2 atmosphere, the labeled MPNST cells that had invaded through the Matrigel were scanned by the bottom reading type fluorescence plate reader (Labsystems Fluoroskan Ascent, Thermo Electron Co., Waltham, MA, USA) at 544/590 nm (absorption/emission). Only the fluorescent cells that migrated through the membrane were detected since the insert membrane blocks fluorescent light. The autofluorescence derived from wells containing medium only was subtracted from the readings obtained from the inserts carrying the fluorescent cells. Means and s.e. of the mean were calculated and statistical differences were analyzed using Student's t-test for non-paired samples. Each assay was performed in triplicate and repeated three times with similar results.
In experiments with phosphorylation inhibitors, after starvation for 48 h in serum-free DMEM/F12, cells were labeled with Dil and preincubated with 0.1–5 μM imatinib or 1–4 μM herbimycin A before PDGF-BB addition. They were then incubated for 48 h in the presence of both PDGF-BB and the inhibitors.
Tumor Tissues
Our study included formalin-fixed, paraffin-embedded sections from 13 cases with MPNST (nine men, four women; age range, 31–82 (mean=53) years) and 15 cases of schwannoma (5 men, 10 women; age range, 22–89 (mean=53.6) years) and 15 cases of neurofibroma (eight men, seven women; age range, 28–75 (mean=58.1) years), diagnosed at the Department of Pathology, Fukuoka University, Japan. Histological diagnosis of MPNST was established based on the widely accepted criteria that require the tumor to conform to one of the following: (1) arises within a peripheral nerve; (2) arises in transition from a benign or other malignant peripheral nerve tumor; (3) develops in a patient with NF1 (von Recklinghausen's disease) and exhibits the same histopathological features as do the majority of MPNSTs arising from nerves; or (4) develops in a patient without NF1, but exhibits histopathological features similar to those seen in most patients with MPNSTs and shows either or both immunohistochemical and ultrastructural features of Schwann or perineural cell differentiation.2
Immunohistochemistry
Immunohistochemical staining was performed using 4-μm-thick paraffin-embedded sections. The tissue sections were deparaffinized in xylene, and rehydrated in a graded series of ethanol solutions and washed in Tris-buffered saline (TBS), pH 7.6. For EGF receptor (EGFR) staining, the tissue samples were digested first with proteinase K (DAKO, Carpinteria, CA, USA) for 5 min at room temperature before staining. After non-specific sites were blocked with 3% BSA and 1% non-fat dry milk in TBS for 10 min at room temperature, the sections were incubated overnight at 4°C with anti-PDGFR-β polyclonal antibody (sc-339, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-EGFR monoclonal antibody (mAb) (H11, DAKO). The sections were then washed in TBS, and incubated with biotinylated goat anti-rabbit (DAKO) or horse anti-mouse (Vector Laboratories, Burlingame, CA, USA) IgG for 30 min at room temperature, followed by streptavidin conjugated to alkaline phosphatase (DAKO) for another 30 min. The reaction was revealed with naphthol AS-BI phosphate (Sigma Chemical Co., St Louis, MO, USA) in 100 ml of 0.2 M TBS (pH 8.2) containing 4% hydrochloric acid and 4% nitric acid and counterstained with Mayer's hematoxylin or methyl green.
The immunohistochemical specificity of the antibody was confirmed by two types of negative controls: substituting mouse (for mAb) or rabbit (for polyclonal antibody) non-immune IgG for the primary antibody and omitting the primary antibody in the staining protocol. The staining results were evaluated semiquantitatively by two independent observers. Immunostaining was considered negative if stained tumor cells were less than 10%. In specimens considered positive, staining of the tumor was quantitated on a scale from 1 to 4 based on the percentage of positive tumor cells. The scale was structured as follows: 1+, 10–25% of cells positive; 2+, 25–50% of cells positive; 3+, 50–75% of cells positive; 4+, >75% of cells positive.
RNA Extraction and RT-PCR Analysis
Total RNA was isolated from fresh frozen tissue of original tumors, FU-SFT8611 and FU-SFT9817 cells using the GlassMAX RNA Microisolation Spin Cartridge System (Gibco), according to the instructions supplied by the manufacturer. Next, 1 μg of total RNA from each sample was reverse-transcribed using Ready-To-Go You-Prime First Strand Beads (GE Healthcare Bio-Sciences, Piscataway, NJ, USA). PCR amplifications were performed with puReTaq Ready-To-Go PCR Beads (GE Healthcare Bio-Sciences). Primer pairs are listed in Table 1. Reaction mixtures were initially denatured at 95°C for 3 min, followed by 35 cycles of 1 min at 95°C, 1 min at the respective annealing temperature (Table 1), and 1 min at 72°C in a thermal cycler (RoboCycler, Stratagene, La Jolla, CA, USA). Finally, 4 μl of the PCR products were analyzed by electrophoresis on 2% agarose gel stained with ethidium bromide.
Detection of Phosphorylated PDGFR by Western Blotting
FU-SFT8611 and FU-SFT9817 cells (4 × 105 cells/well) were seeded in six-well plates in GM. After stabilization of the cells over a 24-h period, the medium was aspirated and replaced with serum-free DMEM/F12 for 48 h. After with or without pretreatment for 60 min with 4 μM herbimycin A or 1–5 μM imatinib, the cells were stimulated for 30 min with 25 ng/ml PDGF-BB in the presence or absence of herbimycin A or imatinib. After removal of the medium, the cells were washed and scraped in phosphate-buffered saline (PBS) and collected by centrifugation at 1000 r.p.m. for 5 min. The cells were then lysed with a cell lysis buffer consisting of 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1 mM Na3VO4, and protease inhibitor cocktail tablets (Complete Mini, Roche Applied Sciences, Penzberg, Germany). Lysed cells were sonicated on ice for 15 s and centrifuged at 16 000 r.p.m. for 20 min at 4°C. The resultant supernatants were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after measurement of their protein concentrations using the Bio-Rad (Hercules, CA, USA) protein assay according to Bradford's method. After electrophoresis, the proteins were transferred electrophoretically to Immobilon membrane (Millipore, Bedford, MA, USA). Non-specific sites were blocked with 2% BSA in 0.05% Tween-20/TBS, pH 7.6 (TBS-T) at 37°C for 1 h and the membrane was incubated overnight at 4°C with antibodies against phospho-PDGFR-β, α-tubulin, phospho-PDGFR-α, PDGFR-α (catalog nos. 3161, 2144, 2992, 3164, respectively), Cell Signaling Technology (Danvers, MA, USA) and PDGFR-β (Santa Cruz) dissolved in TBS-T containing 1% BSA. After washing with TBS-T, the membrane was incubated for 1 h with peroxidase-conjugated goat anti-rabbit IgG. Color was developed with chemiluminescence reagents according to the instructions supplied by the manufacturer (DuPont NEN, Boston, MA, USA). The bands on the film were subjected to image analysis (NIH ImageJ version 1.3719). Statistical analysis was performed using Student's t-test.
Cell Proliferation Assay
Proliferation of MPNST cell lines was evaluated using one-solution cell proliferation assays with the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) (CellTiter 96 Aqueous, Promega, Madison, WI, USA). The MTS compound is bioreduced to formazan by reduced NADPH or reduced NADH produced by metabolically active dehydrogenases of cells, which can be detected at 490 nm. One thousand cells were seeded into 38 mm2 wells of flat-bottomed 96-well plates in triplicate in DMEM/F12 containing 2% FCS in presence or absence of 25 ng/ml of PDGF-BB and/or 5 μM imatinib. The medium was changed every other day. After the treatment, 20 μl of the MTS solution was added to each well and incubated at 37°C for 1 h. The 96-well plate was then placed in a kinetic microplate reader (Benchmark, Bio-Rad) and absorbance was read at 490 nm.
Small Interfering RNA
FU-SFT8611 and 9817 cells were grown to pre-confluence and treated with small interfering RNA (siRNA) for the PDGFR-β (Smart Pool, Dharmacon, Chicago, IL, USA) or control siRNA (B-Bridge International, Sunnyvale, CA, USA) using LipofectAMINE 2000 (Invitrogen) according to the manufacturer's instructions.
DNA Extraction, PCR and DNA Sequencing
Genomic DNA was extracted from the cell lines and frozen tissues by using a DNA STAT-60 (Tel-Test Inc., Friendwood, TX, USA) based on the procedure recommended by the manufacturer. About 50–200 ng of the genomic DNA was amplified using puReTaq Ready-To-Go PCR Beads (GE Healthcare Bio-Sciences). The primer sequences used were as follows; PDGFRB exon 12 forward (F): TGTCCTAGACGGACGAACCT, reverse (R): CCAACTTGAGTCCCCACACT; exon 18 (F): GAAGGGTCTTTCCCCACAAT, (R): CACACTGGTCAGGAGGGAAT.20 Amplicons were 258 and 259 bp, respectively. PDGFRB exons 12 and 18 were analyzed since they correspond to the juxtamembrane and the activation loop coding regions of KIT and PDGFRA.20 The PCR cycling conditions consisted of an initial denaturation step at 94°C for 12 min, following 35 cycles at 94°C for 30 s, 50 s annealing at 57°C, 55 s at 72°C, and final extension for 10 min at 72°C. Negative and β-globin controls were included in every PCR set. The PCR products were purified using a QiAquick PCR purification kit (Qiagen Inc., Hilden, Germany). Direct sequencing of PCR products was performed using Applied Biosystems 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
Results
Profiling Growth Factors that Enhance Matrigel-Invasive Activity of MPNST Cells
First, we examined whether growth factors, reported to be motogenic for tumor cells, could induce Matrigel invasion in two MPNST cell lines by using the fluoroblock invasion assay. The factors tested included PDGF-BB, PDGF-AA, EGF, bFGF, aFGF, IGF, HGF and TGF-β1. These factors are reported to stimulate single-cell locomotion of tumor cells, using Boyden chamber type or scattering assays.21, 22 The concentrations of the above factors used in our study ranged from 0.1 to 100 ng/ml. Among the factors, only PDGF-BB clearly and efficiently induced the Matrigel invasion activities of both FU-SFT8611 and 9817 cells (Figure 1a), whereas EGF (Figure 1c) and HGF (Figure 1d) showed significant induction of invasion by FU-SFT8611 or FU-SFT9817 cells only, respectively. The other factors did not induce significant invasion (Table 2). A relatively broad range of PDGF-BB, from 25 to 100 ng/ml or from 10 to 100 ng/ml, induced Matrigel invasion in FU-SFT8611 and 9817 cells, with a peak response at 50 or 25 ng/ml, respectively (Figure 1a). EGF induced a bell-shaped dose–response curve in FU-SFT8611 cells with a peak response at 1 ng/ml (Figure 1c). PDGF-BB exhibited progressively increasing stimulatory effects up to 72 h of incubation (Figure 1b). A summary of the results of this experiment is shown in Table 2.
Invasive responses of MPNST cell lines FU-SFT9817 and 8611 induced by PDGF-BB (a and b), EGF (c) and HGF (d) by fluoroblock invasion assays. (a, c and d) represent dose–response curves at 48 h, while (b) represents time courses up to 72 h by 50 ng/ml PDGF-BB. Invasive responses are shown as percent of 10% FCS control. Values are mean±s.e. of the mean (n=4 in a–c; n=3 in d). *P<0.01 by Student's t-test.
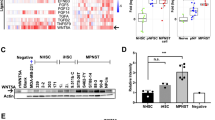
Expression of PDGFR-β and EGFR mRNAs in MPNST
PDGF-BB and EGF activate cells via their cell-surface receptors PDGFR-β and EGFR, respectively.23 In the next step, we examined the expression of PDGFR-β and EGFR mRNA in peripheral nerve sheath tumors including MPNST using reverse transcription-(RT)-PCR. The samples included three MPNST cell lines, eight MPNST tissues, three schwannomas and two neurofibromas. Figure 2 shows a representative example of the results of RT-PCR analysis. All three MPNST cell lines (FU-SFT8611, 9817 and 8710) expressed both PDGFR-β and EGFR mRNA. Seven of the eight MPNST tissues expressed PDGFR-β and five showed EGFR mRNA expression. On the other hand, one of the three schwannomas expressed PDGFR-β mRNA and one expressed EGFR mRNA. Both neurofibroma samples did not express PDGFR-β or EGFR mRNA.
RT-PCR detection of PDGFR-β and EGFR expression in three MPNST cell lines, eight MPNST tissues, three schwannomas and two neurofibromas. MPNST, malignant peripheral nerve sheath tumor; PDGFR-β, platelet-derived growth factor recepto-β; EGFR, epidermal growth factor receptor; G3PDH, glyceraldehyde-3-phosphate dehydrogenase.
Expression of PDGFR-β and EGFR Protein in Peripheral Nerve Sheath Tumors
Expression of PDGFR-β and EGFR protein was examined immunohistochemically using tissue sections of 13 MPNSTs, 2 xenografts in mice of MPNST, 15 schwannomas and 15 neurofibromas. All human tissues and xenografts of MPNST expressed PDGFR-β protein, and EGFR protein was also expressed in all but one xenograft (Table 3). MPNST cells expressed PDGFR-β and EGFR proteins in both cytoplasm and membranes (Figure 3a–c). Higher immunohistochemical positive scores (3+ and 4+) of PDGFR-β and EGFR were noted in 69.2% (9/13) and 46.2% (6/13) of MPNST, respectively. In contrast, none of schwannomas and neurofibromas showed high positive scores (Table 3, Figure 3d). Moreover, xenografts of FU-SFT8611 and 9817 cell lines both retained the immunohistochemical expression scores of their original tumors (Table 3). The low immunohistochemical score of EGFR protein in FU-SFT9817 (Table 3) was consistent with their low responsiveness to EGF stimulation (Figure 1c).
Immunohistochemistry for PDGFR-β and EGFR protein expression in peripheral nerve sheath tumors. (a and b) PDGFR-β expression, (c and d) EGFR expression, (a) FU-SFT8611 xenograft in mice, (b) FU-SFT9817 human tumor tissue, (c) FU-SFT8611 human tumor tissue, (d) neurofibroma (case no. 31). MPNST cells frequently showed diffuse reactivity in the cytoplasm and cell membranes (a–c), while neurofibroma cells are negative (d).
PDGF-BB-Induced Phosphorylation of PDGFR-β and its Blockade by Imatinib Mesylate
On the basis of the expression of PDGFR-β in MPNST tissues and cell lines, we next examined the phosphorylation of PDGFR-β and its inhibition. As shown in Figure 4, exposure of both MPNST cell lines to PDGF-BB (25 ng/ml) for 30 min caused 4 to 5-fold increase in PDGFR-β phosphorylation compared with the basal value, whereas 60 min stimulation caused less phosphorylation levels. PDGF-BB stimulation did not induce any phosphorylation of PDGFR-α. Pretreatment with herbimycin A (4 μM), a broad-spectrum and cell-permeable protein tyrosine kinase inhibitor, decreased PDGF-BB-induced PDGFR-β phosphorylation in both FU-SFT8611 and 9817 by about 97%. We also examined the effect of imatinib mesylate, a specific low-molecular-weight inhibitor of c-Kit and PDGFR tyrosine kinases, on the phosphorylation of PDGFR-β. Pretreatment with imatinib mesylate (5 μM) almost completely suppressed PDGF-BB-induced PDGFR-β phosphorylation in both FU-SFT8611 and FU-SFT9817 (Figure 4, bar graphs).
Effects of herbimycin A and imatinib mesylate on tyrosine phosphorylation of PDGFR-β. MPSNT cell lines were preincubated with herbimycin A or imatinib mesylate at the indicated concentrations for 1 h. The cells were then stimulated with 25 ng/ml of PDGF-BB for 30 min. Equal amounts of lysates were subjected to SDS-PAGE and analyzed by immunoblotting with antibodies to the phosphorylated forms of PDGFR-α and -β. Thereafter, the membrane was reprobed with anti-PDGFR-α and -β and anti-α-tubulin antibodies. The level of phosphorylated PDGFR-β is expressed relative to that of α-tubulin. Values are mean±s.e.m. (n=3).
Effect of Imatinib Mesylate on PDGF-BB-Induced MPNST Cell Invasion
Imatinib and herbimycin A effectively suppressed tyrosine phosphorylation of PDGFR-β as shown above. Thus, we investigated their effects on the invasive activity of MPNST cells. Treatment with herbimycin A (1 μM) suppressed PDGF-BB-induced invasion of Matrigel completely in FU-SFT8611 cells and by about 83.4% in FU-SFT9817 cells (Figure 5a and b). Imatinib mesylate also inhibited the invasion in a dose-dependent manner: treatment with 0.1, 1 and 5 μM imatinib downregulated FU-SFT8611 cell invasion by about 39.9, 43.3 and 86.1%, respectively, and also FU-SFT9817 cell invasion by about 47.1, 54.1 and 79.2%, respectively (Figure 5c and d).
Inhibitory effects of herbimycin A (a and b) and imatinib mesylate (c and d) on MPNST cell invasion (a and c, FU-SFT8611; b and d, FU-SFT9817). MPNST cells were starved for 48 h and labeled with Di1. Then, invasion assays were performed with or without inhibitors for 48 h. Double asterisk represents vehicle control, with equal amounts of DMSO used for dissolving herbimycin A and imatinib. Values are the mean±s.e.m. (n=4). Results are representative of three experiments performed. *P<0.01 by Student's t-test.
Effect of Imatinib Mesylate on MPNST Cell Growth In Vitro
To examine the effect of imatinib mesylate on cell growth, MPNST cells were incubated in the presence or absence of the inhibitor (5 μM) for 8 days. Imatinib mesylate inhibited both 2% FCS- and 2% FCS+PDGF-BB-stimulated cell growth by about 35 and 39% in FU-SFT8611 (Figure 6a) and by 100 and 84.6% in FU-SFT9817 cells, respectively (Figure 6b).
Effects of imatinib mesylate on the growth of MPNST cell lines (a, FU-SFT8611; b, FU-SFT9817). Cells were plated in 96-well plates at a concentration of 8 × 102 (FU-SFT8611) or 1 × 103 (FU-SFT9817) cells/well and grown in DMEM containing 2% FCS in presence (solid triangle) or absence (open triangle) of imatinib mesylate (5 μM), or in DMEM containing 2% FCS+25 ng/ml PDGF-BB in presence (solid circle) or absence (open circle) of imatinib (5 μM). Cell proliferation was determined as described in Materials and methods. Values are the mean±s.e.m. (n=6). Data are representative of four experiments performed. *P<0.01 by Student's t-test.
PDGFR-β Mutations
Three MPNST cell lines (FU-SFT8611, 9817 and 8710) and 10 MPNST tissues (cases no. 1, 3, 5–11, 15 of Table 3) were subjected to DNA sequencing of exons 12 and 18 of PDGFR-β, but no mutations were present in these exons of any of these cell lines or tumors examined.
PDGFR-β Inhibition Blocks MPNST Cell Invasion
To examine the significance of PDGFR-β in MPNST cell invasion, we used siRNA for PDGFR-β. Receptor downregulation was induced by siRNA duplex concentrations greater than 10 nM (Smart pool, Dharmacon) and downregulation was maintainted for 72 h: treatment with 0.01, 0.02 and 0.05 pmol/ml siRNA downregulated PDGFR-β expression by about 80.3, 82.7 and 84.6% at 72 h, respectively (Figure 7a). Similar extents of PDGFR-β downregulation were also observed at 48 h (data not shown). In the presence of siRNA that targets the PDGFR-β, PDGF-BB-stimulated MPNST cell invasion was blocked significantly compared with siRNA control (Figure 7b).
Effects of PDGFR-β siRNA on MPNST cell invasion. FU-SFT8611 cells were transfected with siRNA for PDGFR-β or control siRNA at specified concentrations as described. (a) After 72 h transfection, the cells were lysed and analyzed by Western blotting with anti-PDGFR-α and -β and anti-α-tubulin antibodies. (b) After 48 h transfection (the cells were labeled with Di1 in the last 12 h in the presence of siRNA), the cells were collected and resuspended in serum-free RPMI1640/F12 supplemented with 0.1% BSA and siRNA. Then, invasion assays were performed with or without PDGF-BB (25 ng/ml) for additional 24 h. Values are the mean±s.e.m. (n=4). *P<0.01 by Student's t-test.
Discussion
MPNST is an aggressive malignant tumor, with frequent hematogenous metastasis that determines poor prognosis. Invasion of the vascular basement membrane is a critical step in the hematogenous metastasis. In this study, we identified PDGF-BB as an invasion-inducing factor for MPNST by profiling the motogenic activity of growth factors using Matrigel (basement membrane) invasion assays. This PDGF-BB-induced invasion was dependent on phosphorylation of PDGFR-β in MPNST cells and was effectively inhibited by imatinib mesylate. Imatinib treatment also suppressed MPNST cell growth stimulated by FCS and PDGF-BB. Expression of PDGFR-β was upregulated at both mRNA and protein levels in MPNST tissues compared with benign Schwannomas and neurofibromas. Our study is the first to point PDGF-BB as the invasion-inducing factor for MPNST, the cellular response to which can be suppressed by imatinib treatment, although it is based on in vitro data.
The mechanisms leading to tumorigenesis for MPNST remain unclear. However, it is considered that MPNST cells seem to acquire multiple genetic changes during their progression toward the malignant phenotype, which may be responsible for the aberrant expression of growth factor receptors or their responsiveness.13 In a study with two human MPNST-derived cell lines, PDGF-BB induced a strong proliferative response from the two cell lines, and this mitogenicity of PDGF-BB was transduced essentially through PDGFR-β, which was highly increased in these two cell lines, compared with non-NF1 Schwann cell lines.13 PDGF-BB induced tyrosine phosphorylation of PDGFR-β but not of PDGFR-α, although both types of receptors were expressed in the cells. On the other hand, analysis of differentially expressed genes in plexiform neurofibroma and MPNST from the same NF1-associated MPNST patient identified the PDGFR-α gene as one of the upregulated genes in MPNST.24 In our work, EGF also induced Matrigel invasion in one cell line, and EGFR mRNA and protein were expressed in MPNST cell lines and tissues, a little less frequently than PDGFR. A report described high expression of EGFR in NF1-associated MPNST cell lines.25 It was also shown that elevated EGFR levels through a Src-dependent mechanism caused overexpression of CD44 in MPNST, which have been implicated in tumor cell invasion and metastasis.26 EGF may act as an invasion–inducer in MPNST, but probably less potent than PDGF based on our data. Thus, we narrowed down the target for inhibition to PDGF.
PDGF is a potent mitogen for mesenchymal cells such as dermal fibroblasts, smooth muscle cells, and other connective tissue cells, and plays important roles in various physiologic processes such as embryonic development, wound healing, angiogenesis, hemostasis and platelet aggregation.23 PDGF consists of four chains, A, B, C and D, which dimerize to form at least five hetero- and homodimers, AA, AB, BB, CC and DD. These five forms bind with different affinities to two receptor types, termed α and β. PDGF-A and PDGF-B chains are secreted from the cell as mature ligands, whereas PDGF-C and PDGF-D require proteolytic cleavage outside the cell to become active. The two different subunits of PDGF receptors have different ligand binding specificities; PDGFR-α binds PDGF-A, -B and -C chains, but PDGFR-β binds only PDGF-B and PDGF-D chains.27, 28, 29, 30, 31 In our study, PDGF-BB but not PDGF-AA stimulated MPNST cell invasion of Matrigel. This finding suggests the predominant involvement of PDGFR-β in this PDGF-BB-induced invasion process. This conclusion was supported by selective tyrosine phosphorylation of PDGFR-β in response to PDGF-BB stimulation and the results of studies using siRNA against PDGFR-β.
Imatinib mesylate is a selective low-molecular-weight inhibitor of the PDGF receptor tyrosine kinase and inhibits both PDGF-AA and PDGF-BB-induced receptor phosphorylation.32 In our study, PDGFR-β phosphorylation was less inhibited by 4 μM herbimycin A than by 5 μM imatinb (Figure 4). On the other hand, 1–4 μM herbimycin A blocked MPNST cell invasion totally, while 5 μM of imatinib did not yield a total inhibition of invasion (Figure 5). This may be based on the fact that herbimycin A is a non-specific tyrosine kinase inhibitor and also has a nonselective action on the phosphatidylinositol cycle.33 Herbimycin A may have totally inhibited invasion through inhibition of any other kinases involved in MPNST cell invasion in addition to PDGFR tyrosine kinase. However, this non-specific action can induce adverse effects at the same time. In this regard, imatinib mesylate is more useful as medicine because of its specific activity. Moreover, herbimycin A is not tolerable for the living body.
Previous studies have shown that imatinib mesylate inhibited the constitutively active fusion products arising from the Philadelphia (Ph) chromosome of chronic myelogenous leukemia, and also induced remission in patients with c-Kit positive gastrointestinal stromal tumor (GIST).34, 35, 36 At present, several clinical trials have assessed the usefulness of imatinib mesylate therapy in cancers and sarcomas that express PDGF/PDGFR or Kit ligand/c-Kit. For example, imatinib treatment was effective, at least in part, against soft tissue tumors such as Kaposi's sarcoma, aggressive fibromatosis (AF, desmoid tumor) and dermatofibrosarcoma protuberans (DFSP).37, 38, 39, 40, 41 In AF patients, it was suggested that imatinib response was probably mediated by inhibition of PDGFR-β kinase activity.38, 39 However, mutations of PDGFR-β was not detected in the AF specimens. In DFSP with t(17;22), the translocation results in transcriptional upregulation of the PDGFB gene, in the form of a COL1A1 (collagen 1 alpha 1)-PDGFB fusion oncogene.40, 41 In our study, neither the two MPNST cell lines nor the human MPNST tissues examined demonstrated mutation of the PDGFR-β gene, exons 12 and 18. Moreover, our two cell lines displayed complex karyotypes with no common abnormalities, and no definite translocation involving PDGF and PDGFR genes was demonstrated by karyotype analysis.42 While highly complex and aberrant karyotypes containing numerical and structural changes have been described for NF1-associated and sporadic MPNST, no consistent imbalances have been reported.43, 44 PDGFR-β mutation has never been described in MPNST, while PDGFR-α mutation was detected in seven tumors from six MPNST patients.45
In our study, invasion and growth of both MPNST cell lines were inhibited in vitro by treatment with 0.1–5 or 5 μM imatinib mesylate, respectively (Figures 5 and 6). Growth inhibition of MPNST cell culture S462 by treatment with 2 and 10 μM imatinib mesylate was also recently reported.45 Within the living body, imatinib mesylate is rapidly absorbed after oral administration, and the mean peak concentration of 4.6 μM (2.3 μg/ml) is recorded following once-daily administration of 400 mg of imatinib mesylate. The mean plasma concentration is 1.46 μM (0.72 μg/ml) 24 h after the administration of 400 mg of imatinib mesylate at steady state. Imatinib mesylate is well tolerable, although the maximum tolerable dose was not identified. Therefore, 600–800 mg of imatinib mesylate/day administered orally is commonly used.34, 46 Considered together, the above studies suggest that suppression of tumor invasion and growth with 0.1–5 μM imatinib mesylate seems promising, although further in vivo studies of animal models are needed to confirm the results. Such studies are currently being conducted in our laboratory.
In conclusion, our study has shown that imatinib mesylate inhibits PDGF-BB-induced invasion and proliferation of MPNST cells by suppressing phosphorylation of PDGFR-β. The effectiveness of imatinib mesylate in vitro suggests that targeting PDGFR-β may result in the establishment of novel treatments for MPNST. These results encourage further preclinical investigations using imatinib mesylate or current and developed novel strategies to target PDGFR-α and PDGFR-β, which may be of great value in MPNST treatment.
References
Lewis JJ, Brennan MF . Soft tissue sarcomas. Curr Probl Surg 1996;33:817–872.
Scheithauer BW . Malignant peripheral nerve sheath tumor (MPNST). Tumor of the peripheral nervous system. Atlas of Tumor Pathology, third series. Armed Forces Institute of Pathology: Washington, DC, 1999.
Woodruff JM . Pathology of major peripheral nerve sheath tumors. Soft Tissue Tumors (International Academy of Pathology Monograph). Williams and Wilkins: Baltimore, 1996.
Ducatman BS, Scheithauer BW, Piepgras DG, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986;57:2006–2021.
Wong WW, Hirose T, Scheithauer BW, et al. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys 1998;42:351–360.
Wanebo JE, Malik JM, VandenBerg SR, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer 1993;71:1247–1253.
Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–1074.
Davis JB, Stroobant P . Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol 1990;110:1353–1360.
Eccleston PA, Funa K, Heldin CH . Expression of platelet-derived growth factor (PDGF) and PDGF alpha- and beta-receptors in the peripheral nervous system: an analysis of sciatic nerve and dorsal root ganglia. Dev Biol 1993;155:459–470.
Ridley AJ, Davis JB, Stroobant P, et al. Transforming growth factors-beta 1 and beta 2 are mitogens for rat Schwann cells. J Cell Biol 1989;109:3419–3424.
Krasnoselsky A, Massay MJ, DeFrances MC, et al. Hepatocyte growth factor is a mitogen for Schwann cells and is present in neurofibromas. J Neurosci 1994;14:7284–7290.
Ratner N, Lieberman MA, Riccardi VM, et al. Mitogen accumulation in von Recklinghausen neurofibromatosis. Ann Neurol 1990;27:298–303.
Badache A, De Vries GH . Neurofibrosarcoma-derived Schwann cells overexpress platelet-derived growth factor (PDGF) receptors and are induced to proliferate by PDGF BB. J Cell Physiol 1998;177:334–342.
Watanabe T, Oda Y, Tamiya S, et al. Malignant peripheral nerve sheath tumour arising within neurofibroma. An immunohistochemical analysis in the comparison between benign and malignant components. J Clin Pathol 2001;54:631–636.
Dang I, DeVries GH . Schwann cell lines derived from malignant peripheral nerve sheath tumors respond abnormally to platelet-derived growth factor-BB. J Neurosci Res 2005;79:318–328.
Su W, Gutmann DH, Perry A, et al. CD44-independent hepatocyte growth factor/c-Met autocrine loop promotes malignant peripheral nerve sheath tumor cell invasion in vitro. Glia 2004;45:297–306.
Aoki M, Nabeshima K, Nishio J, et al. Establishment of three malignant peripheral nerve sheath tumor cell lines, FU-SFT8611, 8710 and 9817: conventional and molecular cytogenetic characterization. Int J Oncol 2006;29:1421–1428.
Shulby SA, Dolloff NG, Stearns ME, et al. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res 2004;64:4693–4698.
ImageJ: Image processing and analysis in Java. http://rsb.info.nih.gov/ij/.
Sihto H, Franssila K, Tanner M, et al. Platelet-derived growth factor receptor family mutations in gastrointestinal stromal tumours. Scand J Gastroenterol 2006;41:805–811.
Stoker M, Gherardi E . Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta 1991;1072:81–102.
Nabeshima K, Inoue T, Kataoka H, et al. Cohort migration of cancer cells. Connective Tissue 1997;29:199–212.
Heldin CH, Westermark B . Growth factors: mechanism of action and relation to oncogenes. Cell 1984;37:9–20.
Holtkamp N, Mautner VF, Friedrich RE, et al. Differentially expressed genes in neurofibromatosis 1-associated neurofibromas and malignant peripheral nerve sheath tumors. Acta Neuropathol (Berlin) 2004;107:159–168.
DeClue JE, Heffelfinger S, Benvenuto G, et al. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest 2000;105:1233–1241.
Su W, Sin M, Darrow A, et al. Malignant peripheral nerve sheath tumor cell invasion is facilitated by Src and aberrant CD44 expression. Glia 2003;42:350–358.
Hart CE, Forstrom JW, Kelly JD, et al. Two classes of PDGF receptor recognize different isoforms of PDGF. Science 1988;240:1529–1531.
Heldin CH, Ostman A, Ronnstrand L . Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1998;1378:F79–F113.
Li X, Ponten A, Aase K, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol 2000;2:302–309.
Bergsten E, Uutela M, Li X, et al. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol 2001;3:512–516.
Li X, Eriksson U . Novel PDGF family members: PDGF-C and PDGF-D. Cytokine Growth Factor Rev 2003;14:91–98.
Buchdunger E, Cioffi CL, Law N, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 2000;295:139–145.
Santini F, Beaven MA . Nonselective actions of herbimycin A. Immunol Today 1993;14:369–370.
Savage DG, Antman KH . Imatinib mesylate—a new oral targeted therapy. N Engl J Med 2002;346:683–693.
Capdeville R, Buchdunger E, Zimmermann J, et al. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov 2002;1:493–502.
Heinrich MC, Blanke CD, Druker BJ, et al. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol 2002;20:1692–1703.
Koon HB, Bubley GJ, Pantanowitz L, et al. Imatinib-induced regression of AIDS-related Kaposi's sarcoma. J Clin Oncol 2005;23:982–989.
Mace J, Sybil Biermann J, Sondak V, et al. Response of extraabdominal desmoid tumors to therapy with imatinib mesylate. Cancer 2002;95:2373–2379.
Heinrich MC, McArthur GA, Demetri GD, et al. Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J Clin Oncol 2006;24:1195–1203.
McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol 2005;23:866–873.
Shimizu A, O'Brien KP, Sjoblom T, et al. The dermatofibrosarcoma protuberans-associated collagen type Ialpha1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res 1999;59:3719–3723.
Aoki M, Nabeshima K, Nishio J, et al. Establishment of three malignant peripheral nerve sheath tumor cell lines, FU-SFT8611, 8710 and 9817: conventional and molecular cytogenetic characterization. Int J Oncol 2006;29:1421–1428.
Mertens F, Rydholm A, Bauer HF, et al. Cytogenetic findings in malignant peripheral nerve sheath tumors. Int J Cancer 1995;61:793–798.
Schmidt H, Taubert H, Wurl P, et al. Cytogenetic characterization of six malignant peripheral nerve sheath tumors: comparison of karyotyping and comparative genomic hybridization. Cancer Genet Cytogenet 2001;128:14–23.
Holtkamp N, Okuducu AF, Mucha J, et al. Mutation and expression of PDGFRA and KIT in malignant peripheral nerve sheath tumors, and its implications for imatinib sensitivity. Carcinogenesis 2006;27:664–671.
Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1031–1037.
Acknowledgements
We thank Dr J Nishio, Department of Orthopedics, Fukuoka University Chikushi Hospital, for the kind and encouraging discussion and Ms M Ishiguro, C Fujita and M Onitsuka for their kind help in tissue culture and immunohistochemistry. This work was supported in part by a Grant-in-Aid for Scientific Research (15390119) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a Grant-in-Aid for Research Project from Central Research Institute, Fukuoka University School of Medicine (041001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aoki, M., Nabeshima, K., Koga, K. et al. Imatinib mesylate inhibits cell invasion of malignant peripheral nerve sheath tumor induced by platelet-derived growth factor-BB. Lab Invest 87, 767–779 (2007). https://doi.org/10.1038/labinvest.3700591
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3700591
Keywords
This article is cited by
-
Curse of the devil: molecular insights into the emergence of transmissible cancers in the Tasmanian devil (Sarcophilus harrisii)
Cellular and Molecular Life Sciences (2020)
-
Overexpression of PDGFRA cooperates with loss of NF1 and p53 to accelerate the molecular pathogenesis of malignant peripheral nerve sheath tumors
Oncogene (2017)
-
Imatinib mesylate inhibits cell growth of malignant peripheral nerve sheath tumors in vitro and in vivothrough suppression of PDGFR-β
BMC Cancer (2013)
-
Expression and role of GLUT-1, MCT-1, and MCT-4 in malignant pleural mesothelioma
Virchows Archiv (2013)
-
The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma
Nature Reviews Gastroenterology & Hepatology (2012)