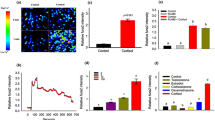
Abstract
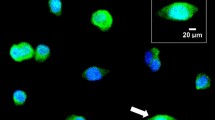
CALRETICULIN is a multifunctional protein that acts as a major Ca2+-binding (storage) protein in the lumen of the endoplasmic reticulum1. It is also found in the nucleus2, suggesting that it may have a role in transcription regulation. Calreticulin has been reported to bind to the synthetic peptide KLGFFKR3, which is almost identical to an amino-acid sequence in the DNA-binding domain of the superfamily of nuclear receptors4–6. Could calreticulin interact with the DNA-binding domain of these receptors and affect their function? Here we report that the amino terminus of calreticulin interacts with the DNA-binding domain of the glucocorticoid receptor and prevents the receptor from binding to its specific glucocorticoid response element. Overexpression of calreticulin in mouse L fibroblasts inhibits glucocorticoid-response-mediated transcriptional activation of a glucocorticoid-sensitive reporter gene and of the endogenous, glucocorticoid-sensitive gene encoding cytochrome P450. Together these results indicate that calreticulin may be important in gene transcription, regulating the glucocorticoid receptor and perhaps other members of the super-family of nuclear receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
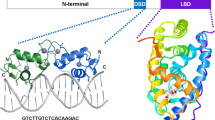
References
Michalak, M., Milner, R. E., Burns, K. & Opas, M. Biochem. J. 285, 681–692 (1992).
Opas, M., Dziak, E., Fliegel, L. & Michalak, M. J. Cell. Physiol. 149, 160–171 (1991).
Rojiani, M. V., Finley, B. B., Gray, V. & Dedhar, S. Biochemistry 30, 9859–9865 (1991).
Evans, R. M. Science 240, 889–895 (1988).
Beato, M. Cell 56, 335–344 (1989).
Laudet, V., Hänni, C., Coll, J., Catzeflis, F. & Stehelin, D. EMBO J. 11, 1103–1013 (1992).
Luisi, B. F. et al. Nature 352, 497–505 (1991).
Drouin, J. et al. Molec. Endocrinol. 6, 1299–1309 (1992).
Dedhar, S. et al. Nature 367, 480–483 (1994).
Baksh, S. & Michalak, M. J. biol. Chem. 266, 21458–21465 (1991).
Hollenberg, S. M. et al. Nature 318, 635–641 (1985).
Laemmli, U. K. Nature 227, 680–685 (1970).
Argentin, S., Sun, Y. L., Lihrmann, I., Schmidt, T. J., Drouin, J. & Nemer, M. J. biol. Chem. 266, 23315–23322 (1991).
Milner, R. E. et al. J. biol. Chem. 266, 7155–7165 (1991).
Burns, K., Helgason, C. D., Bleackley, R. C. & Michalak, M. J. biol. Chem. 267, 19039–19042 (1992).
deWet, J. R., Wood, K. V., Deluca, M., Helinski, D. R. & Subramani, S. Molec. cell. Biol. 7, 725–737 (1987).
Edwards, R., Murray, B. P. & Boobies, A. R. Meth. Enzym. 206, 220–233 (1991).
Hardwick, J. P., Gonzalez, F. J. & Kasper, C. B. J. biol. Chem. 258, 10182–10186 (1983).
Fliegel, L., Burns, K., MacLennan, D. H., Reithmeier, R. A. F. & Michalak, M. J. biol. Chem. 264, 21522–21528 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burns, K., Duggan, B., Atkinson, E. et al. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature 367, 476–480 (1994). https://doi.org/10.1038/367476a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/367476a0
This article is cited by
-
Calreticulin: a quintessential multifaceted protein with therapeutic potential
Journal of Proteins and Proteomics (2023)
-
A transgenic mouse line for assaying tissue-specific changes in endoplasmic reticulum proteostasis
Transgenic Research (2023)
-
Calreticulin enhances gastric cancer metastasis by dimethylating H3K9 in the E-cadherin promoter region mediating by G9a
Oncogenesis (2022)
-
Calreticulin Ins5 and Del52 mutations impair unfolded protein and oxidative stress responses in K562 cells expressing CALR mutants
Scientific Reports (2019)
-
Calreticulin regulates MYCN expression to control neuronal differentiation and stemness of neuroblastoma
Journal of Molecular Medicine (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.