Abstract
The catalytic γ-subunit of the enzyme phosphatidylinositol-3-OH kinase (PI(3)Kγ) relays signals from G- protein-coupled receptors at the cell membrane and mediates leukocyte responses to chemokines and chemoattractants1,2,3. Sasaki et al.4 report that mice that cannot produce PI(3)Kγ have a high incidence of colorectal carcinomas, causing weight loss and premature death. However, PI(3)Kγ-null mouse strains have been independently generated in three other laboratories; none of these mice developed tumours and their weight and lifespan were normal. This casts coubt on the idea that loss of functional PI(3)Kγ leads directly to transformation of colon epithelial cells and tumour progression.
Similar content being viewed by others
Main
Disrupting signalling by chemokine receptors has been considered as a strategy to fight chronic inflammatory disease. Signals from these receptors are integrated by PI(3)Kγ5,6, whose crystal structure7 and inhibitor interactions8 are understood in detail, paving the way to rapid therapeutic exploitation of PI(3)Kγ as a drug target. But promising research was interrupted by the claim of Sasaki et al.4 that loss of functional PI(3)Kγ causes colon cancer in mice.
Sasaki et al.4 base their conclusions on the fact that their PI(3)Kγ-null mouse strain rapidly developed colorectal carcinomas. Using total colon and mucosal samples, they detected PI(3)Kγ in colon tissue but not in murine or human colorectal adenocarcinomas, inferring that the loss of PI(3)Kγ was crucial to the transformation process in epithelial cells.
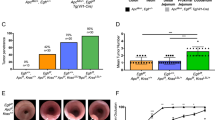
The murine PI(3)Kγ gene was independently inactivated by four groups, including ourselves1,2,3 and B.L. et al. (unpublished observations), using four different strategies (Fig. 1a), all of which confirmed that this enzyme is important for transmission of inflammatory signals. In our studies, however, mice lacking PI(3)Kγ did not develop tumours, or succumb to weight loss and premature death (Fig. 1b). Analysis of tissue biopsies from more than 100 PI(3)Kγ-null mice at various ages and of both sexes from two genetic backgrounds (129/Sv inbred and C57BL/6J/129 outbred) showed no malignant transformation (results not shown).
a, The wild-type PI(3)Kγ allele (WT) was targeted using four strategies. Allele I: interception1 of exon 2 (embryonic stem cells and mouse strains indicated; for details of the PI(3)Kγ gene, see ref. 9); allele II: deletion of exon 2, as carried out by Sasaki et al.2,4; direction of transcription of Neo is opposite to that of PI(3)Kγ; allele III: fusion3 of PI(3)Kγ to green fluorescent protein (GFP) and excision of exon 2; allele IV: start of exon 2 deleted (˜350 base pairs; B.L. et al., unpublished observations). b, Weight and mortality of normal (filled symbols; black lines) and PI(3)Kγ−/− (allele I: white symbols; blue lines) mice; 465 WT and 602 PI(3)Kγ−/− animals; age, 2 yr; sex ratio, 9/10 WT and 34/8 PI(3)Kγ−/− females/males; mortality from Sasaki et al.4 shown in red). c, Murine WT (+) and PI(3)Kγ−/− (–) splenocytes (spl.), total colon, mechanically sheared mucosa and isolated colon epithelial cells were probed for the leukocyte marker CD18, PI(3)Kγ (mouse monoclonal anti-PI(3)Kγ, amino-terminal epitope), and the pan-epithelial marker Lu-5. d, Anti-PI(3)Kγ and Lu-5 western blots of total human cell lysates from neutrophils (NØ), retinoic-acid-differentiated HL60, primary cultures of normal human colonocytes10 from large bowel resected from four patients with diverticulitis, and the HT29-Cl.16E cell line. e, PI(3)Kγ immunoreactivity (red) in rat colonic mucosa (inset, cross-section of crypt). f, Invasion of collagen gels and serum-withdrawal-induced apoptosis of human colorectal HCT8/S11 cells stably transfected with control vector (C), membrane-targeted PI(3)Kγ (PI(3)Kγ–CAAX, black bars11) or a catalytically inactive mutant (PI(3)Kγ (K833R)–CAAX; KR–CAAX, white bars). The PI(3)K inhibitor LY294002 (LY) was used at 10 μM. Further details are available at http://www.unifr.ch/biochem/wymann.
We therefore re-examined the PI(3)Kγ-expression pattern reported by Sasaki et al.4, and found that PI(3)Kγ signals in colonic mucosa correlate with the presence of leukocytes, as shown by the CD18 marker or by histology, but that PI(3)Kγ is undetectable in normal colonic epithelial cells (positive for the Lu5 cytokeratin marker) from mice, human patients or rats (Fig. 1c–e). We conclude that normal and transformed colonic epithelial cells (such as the HT29 cancer cell line) do not express detectable amounts of PI(3)Kγ, making a direct cause-and-effect relationship between loss of PI(3)Kγ and development of colon cancer unlikely.
Invasiveness and growth-factor-in-dependent survival of human colorectal HCT8/S11 tumour cells was promoted by constitutively active, membrane-targeted PI(3)Kγ (PI(3)Kγ–CAAX), but not by its absence or by stable transfection with catalytically inactive PI(3)Kγ (KR–CAAX; Fig. 1f). This suggests that malignancy is coupled to activated PI(3)K and not to its loss.
Our findings are consistent with a lack of tumorigenesis in PI(3)Kγ-null strains generated by three out of four strategies. The reproducibility and consistency of the diverging results, however, make it possible that an unknown gene-targeting effect enhances other growth-promoting signals in the PI(3)Kγ-null allele used by Sasaki et al.4. Their interesting phenotype therefore needs further investigation, and may eventually reveal an important cause of colon cancer.
References
Hirsch, E. et al. Science 287, 1049–1053 (2000).
Sasaki, T. et al. Science 287, 1040–1046 (2000).
Li, Z. et al. Science 287, 1046–1049 (2000).
Sasaki, T. et al. Nature 406, 897–902 (2000).
Wymann, M. P., Sozzani, S., Altruda, F., Mantovani, A. & Hirsch, E. Immunol. Today 21, 260–264 (2000).
Rickert, P., Weiner, O. D., Wang, F., Bourne, H. R. & Servant, G. Trends Cell Biol. 10, 466–473 (2000).
Walker, E. H., Perisic, O., Ried, C., Stephens, L. & Williams, R. L. Nature 402, 313–320 (1999).
Walker, H. E. et al. Mol. Cell 6, 909–919 (2000).
Hirsch, E. et al. Gene 256, 69–81 (2000).
Branka, J. E. et al. Gastroenterology 112, 1887–1894 (1997).
Bondeva, T. et al. Science 282, 293–296 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbier, M., Attoub, S., Calvez, R. et al. Weakening link to colorectal cancer?. Nature 413, 796 (2001). https://doi.org/10.1038/35101660
Issue Date:
DOI: https://doi.org/10.1038/35101660
This article is cited by
-
Synergism between altered cortical polarity and the PI3K/TOR pathway in the suppression of tumour growth
EMBO reports (2012)
-
Studies on the Expression Patterns of Class I PI3K Catalytic Subunits and Its Prognostic Significance in Colorectal Cancer
Cell Biochemistry and Biophysics (2012)
-
Targeting PI3K in neuroblastoma
Journal of Cancer Research and Clinical Oncology (2010)
-
Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis
Oncogene (2007)
-
Prognosis of stage II colon cancer by non-neoplastic mucosa gene expression profiling
Oncogene (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.