Abstract
Seismological studies have revealed that a complex texture or heterogeneity exists in the Earth's inner core and at the boundary between core and mantle1,2,3,4. These studies highlight the importance of understanding the properties of iron when modelling the composition and dynamics of the core and the interaction of the core with the lowermost mantle5,6,7. One of the main problems in inferring the composition of the lowermost mantle is our lack of knowledge of the high-pressure and high-temperature chemical reactions that occur between iron and the complex Mg–Fe–Si–Al-oxides which are thought to form the bulk of the Earth's lower mantle. A number of studies6,8,9,10,11,12 have demonstrated that iron can react with MgSiO3-perovskite at high pressures and high temperatures, and it was proposed6,8 that the chemical nature of this process involves the reduction of silicon by the more electropositive iron. Here we present a study of the interaction between iron and corundum (Al2O3) in electrically- and laser-heated diamond anvil cells at 2,000–2,200 K and pressures up to 70 GPa, simulating conditions in the Earth's deep interior. We found that at pressures above 60 GPa and temperatures of 2,200 K, iron and corundum react to form iron oxide and an iron–aluminium alloy. Our results demonstrate that iron is able to reduce aluminium out of oxides at core–mantle boundary conditions, which could provide an additional source of light elements in the Earth's core and produce significant heterogeneity at the core–mantle boundary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Vidale, J. E. & Earle, P. S. Fine-scale heterogeneity in the Earth's inner core. Nature 404, 273–275 (2000).
Vidale, J. E., Dodge, D. A. & Earle, P. S. Slow differential rotation of the Earth's inner core indicated by temporal changes in scattering. Nature 405, 445–448 (2000).
Breger, L., Romanowicz, B. & Rousset, S. New constraints on the structure of the inner core from P′P′. Geophys. Res. Lett. 27, 2781–2784 (2000).
Carnero, E. J. & Jeanloz, R. Fuzzy patches on the Earth's core–mantle boundary? Geophys. Res. Lett. 27, 2777–2780 (2000).
Stixrude, L. & Brown, J. M. The Earth's core. Rev. Mineral. 37, 262–283 (1998).
Knittle, E. & Jeanloz, R. Simulating the core–mantle boundary: an experimental study of high-pressure reactions between silicates and liquid iron. Geophys. Res. Lett. 16, 609–612 (1989).
Poirier, J. P., Malavergne, V. & Le Mouël, J. L. in The Core–Mantle Boundary Region (eds Gurnis, M., Wysession, M. E., Knittle, E. & Buffet, B. A.) 131–137 (AGU, Washington, 1998).
Knittle, E. & Jeanloz, R. Earth's core–mantle boundary: Results of experiments at high pressures and high temperatures. Science 251, 1438–1443 (1991).
Goarant, F., Guyot, F., Peyroneau, J. & Poirier, J. P. High-pressure and high-temperature reactions between silicates and liquid iron alloys in the diamond anvil cell, studied by analytical electron microscopy. J. Geophys. Res. 97, 4477–4487 (1992).
Song, X. & Ahrens, T. J. Pressure-temperature range of reactions between liquid iron in the outer core and mantle silicates. Geophys. Res. Lett. 21, 153–156 (1994).
Malavergne, V. Etude experimentale à haute pression et haute température du partage des éléments de transition entre les phases du manteau et du noyau de la Terre. Thesis, Univ. Paris 7, (1996).
Tschauner, O. et al. Partitioning of nickel and cobalt between silicate perovskite and metal at pressures up to 80 GPa. Nature 398, 604–607 (1999).
Anderson, D. L. Theory of the Earth (Blackwall Scientific, Boston, 1989).
Allègre, C. J., Poirier, J.-P., Humler, E. & Hofmann, A. W. The chemical composition of the Earth. Earth Planet. Sci. Lett. 134, 515–526 (1995).
Ruff, L. J. & Anderson, D. L. Core formation, evolution, and convection; a geophysical model. Phys. Earth Planet. Inter. 21, 181–201 (1980).
McCammon, C. A. Perovskite as a possible sink for ferric iron in the lower mantle. Nature 387, 694–696 (1997).
Lauterbach, S., McCammon, C. A., van Aken, P., Langenhorst, F. & Seifert, F. Mössbauer and ELNES spectroscopy of (Mg,Fe)(Si,Al)O3 perovskite: a highly oxidised component of the lower mantle. Contrib. Mineral. Petrol. 138, 17–26 (2000).
Hillgren, V. J., Drake, M. J. & Rubie, D. C. High-pressure and high-temperature experiments on core–mantle segregation in the accreting Earth. Science 264, 1441–1445 (1994).
Li, J. & Agee, C. B. Geochemistry of mantle–core differentiation at high pressure. Nature 381, 686–689 (1996).
Fabrichnaya, O. B. & Sundman, B. The assessment of thermodynamic parameters in the Fe–O and Fe–Si–O systems. Cosmochim. Acta 61, 4539–4555 (1997).
Dubrovinsky, L. S., Saxena, S. K., Lazor, P. & Weber, H.-P. Structure of β-iron at high temperature and pressure. Science 281, 11–12 (1998).
Yoo, C. S., Akella, J., Campbell, A. J., Mao, H. K. & Hemley, R. J. Phase diagram of iron by in situ X-ray diffraction: implications for Earth's core. Science 270, 1473–1475 (1995).
El Goresy, A., Dubrovinsky, L., Sharp, T. G. & Chen, M. A new monoclinic post-stishovite polymorph of silica in the SNC meteorite Shergotty. Science 288, 1632–1634 (2000).
Dubrovinsky, L. S. et al. Stability of ferropericlase in the lower mantle. Science 289, 430–432 (2000).
Dubrovinsky, L. S. & Saxena, S. K. Emissivity measurements on same metals and oxides using multiwavelength spectral radiometry. High Pressure High Temp. 31, 393–399 (1999).
Mao, H. K., Bell, P. M. & Hadidiacos, C. in High-Pressure Research in Mineral Physics (eds Manghnani, M. H. & Syono, Y.) 135–138 (Terra Scientific, Tokyo, and American Geophysical Union, Washington DC, 1987).
Dubrovinsky, L. S., Saxena, S. K. & Lazor, P. High-pressure and high-temperature in situ x-ray diffraction study of iron and corundum to 68 GPa using internally heated diamond anvil cell. Phys. Chem. Minerals 25, 434–441 (1998).
Dubrovinskaia, N. A., Dubrovinsky, L. S., Karlsson, A., Saxena, S. K. & Sundman, B. Experimental study of thermal expansion and phase transformations in iron-rich Fe–Al alloys. Calphad 23, 69–84 (1999).
Pasternak, M. P. et al. High pressure collapse of magnetism in Fe0.94O: Mössbauer spectroscopy beyond 100 GPa. Phys. Rev. Lett. 79, 5046–5049 (1997).
Acknowledgements
We thank D. C. Rubie for comments and discussions. This work was supported by the Swedish Science Foundation (NFR), the Wallenberg and Göran Gustafssons Stiftelse Funds.
Author information
Authors and Affiliations
Corresponding author
Supplementary information

Figure 1a
(JPG 72.4 KB)
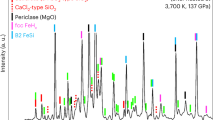
Diffraction pattern of the sample electrically heated at 59(2) GPa and 1450(50) K processed with GSAS program. The pattern is completely explained as a mixture of two phases – corundum (lower ticks, a=4.4966(6) Å, c=12.286(2) Å) and e -Fe (upper ticks, a=2.3606(3) Å, c=3.7719(4) Å). Background is subtracted.

Figure 1b
(JPG 64.7 KB)
Diffraction pattern of the sample electrically heated at 63(5) GPa and 2200(50) K processed with GSAS program. The pattern could not be described just as a mixture (b) of corundum and e -Fe, but a mixture (c) of corundum (lower ticks, a=4.4915(6) Å, c=12.256(2) Å), e -Fe (middle ticks, a=2.3533(2) Å, c=3.7564(4) Å), and rhombohedral wustite (upper ticks, a=2.843(1) Å, a =58.56(2)o) explain the pattern. Background is subtracted.

Figure 1c
(JPG 68.5 KB)
Diffraction pattern of the sample electrically heated at 63(5) GPa and 2200(50) K processed with GSAS program. The pattern could not be described just as a mixture (b) of corundum and e -Fe, but a mixture (c) of corundum (lower ticks, a=4.4915(6) Å, c=12.256(2) Å), e -Fe (middle ticks, a=2.3533(2) Å, c=3.7564(4) Å), and rhombohedral wustite (upper ticks, a=2.843(1) Å, a =58.56(2)o) explain the pattern. Background is subtracted.

Figure 1d
(JPG 31.7 KB)
Diffraction pattern of the sample laser-heated at 56(2) GPa and 2000(150) K, collected at ID30 beam line at ESRF. Blue bars show calculated intensities of the wustite reflections, and red ones - intensities of Fe3Al reflections.

Figure 2a
(JPG 55 KB)
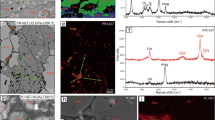
Schematic diagram of the high-pressure assemblage used for microprobe analysis of the product of the reaction between iron and corundum. Specially shaped stainless steel gasket of 300 m m thickens was indented to 50 m m between diamonds with 300 m m culets. Pure corundum was loaded in to the indentation, covered by a 2 m m thick iron foil and NaCl with several small ruby chips were placed on the top. Pressure was measured by ruby fluorescence. The sample was heated by a Nd:YAG laser at pressures between 68 and 74 GPa and temperatures between 2200 and 2350 K. After complete decompression NaCl was washed out and the chemical composition of the heated foil was analysed.

Figure 2b
(JPG 169 KB)
Backscattered electron images of the recovered sample (dark color corresponds to iron-rich, and light area – to aluminum-rich parts). White circle marks indentation. Chemical composition of heated part of the foil varied from 2 wt.% Al to 10 wt.% Al in good agreement with X-ray data. White areas corresponds to higher Al content, and dark one to higher Fe content. Chemical analyses were obtained using the CAMECA SX-50 electron microprobe, employing a PAP correction program. The operating conditions were: accelerating voltage 20 kV, beam current 50 nA, and 1-2 m m beam diameter. Penetration depth of the beam is less than 2 m m. Counting times were 10 s. The following analyzing crystals were used: TAP for Al Ka and LiF for Fe Ka.
Rights and permissions
About this article
Cite this article
Dubrovinsky, L., Annersten, H., Dubrovinskaia, N. et al. Chemical interaction of Fe and Al2O3 as a source of heterogeneity at the Earth's core–mantle boundary. Nature 412, 527–529 (2001). https://doi.org/10.1038/35087559
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35087559
This article is cited by
-
Chemical Interactions of Alumina–Carbon Refractories with Molten Steel at 1823 K (1550 °C): Implications for Refractory Degradation and Steel Quality
Metallurgical and Materials Transactions B (2011)
-
Iron–silica interaction at extreme conditions and the electrically conducting layer at the base of Earth's mantle
Nature (2003)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.