Abstract
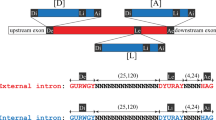
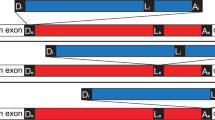
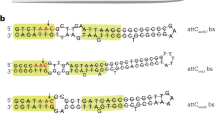
Self-splicing group II introns may be the evolutionary progenitors of eukaryotic spliceosomal introns1,2,3,4,5,6,7, but the route by which they invade new chromosomal sites is unknown. To address the mechanism by which group II introns are disseminated, we have studied the bacterial Ll.LtrB intron from Lactococcus lactis8. The protein product of this intron, LtrA, possesses maturase, reverse transcriptase and endonuclease enzymatic activities9,10,11. Together with the intron, LtrA forms a ribonucleoprotein (RNP) complex which mediates a process known as retrohoming11. In retrohoming, the intron reverse splices into a cognate intronless DNA site. Integration of a DNA copy of the intron is recombinase independent but requires all three activities of LtrA11. Here we report the first experimental demonstration of a group II intron invading ectopic chromosomal sites, which occurs by a distinct retrotransposition mechanism. This retrotransposition process is endonuclease-independent and recombinase-dependent, and is likely to involve reverse splicing of the intron RNA into cellular RNA targets. These retrotranspositions suggest a mechanism by which splicesomal introns may have become widely dispersed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jacquier,A. Self-splicing group II and nuclear pre-mRNA introns: how similar are they? Trends Biochem. Sci. 15, 351– 354 (1990).
Sharp,P. A. Five easy pieces. Science 254, 663 (1991).
Cavalier-Smith,T. Intron phylogeny: a new hypothesis. Trends Genet. 7 , 145–148 (1991).
Roger,A. J. & Doolittle,W. F. Why introns-in-pieces? Nature 364, 289–290 ( 1993).
Weiner,A. M. mRNA splicing and autocatalytic introns: distant cousins or the products of chemical determinism? Cell 72, 161– 164 (1993).
Michel,F. & Ferat,J. Structure and activities of group II introns. Annu. Rev. Biochem. 64, 435– 461 (1995).
Hetzer,M., Wurzer,G., Schweyen,R. J. & Mueller,M. W. Trans-activation of group II intron splicing by nuclear U5 snRNA. Nature 386, 417–420 ( 1997).
Mills,D. A., Manias,D. A., McKay,L. L. & Dunny,G. M. Homing of a group II intron from Lactococcus lactis subsp. lactis ML3. J. Bacteriol. 179, 6107– 6111 (1997).
Lambowitz,A. M., Caprara,M. G., Zimmerly,S. & Perlman,P. S. in The RNA World 451–485 (Cold Spring Harbor Laboratory Press, New York, 1999).
Matsuura,M. et al. A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: biochemical demonstration of maturase activity and insertion of new genetic information within the intron. Genes Dev. 11, 2910–2924 (1997).
Cousineau,B. et al. Retrohoming of a bacterial group II intron: mobility via complete reverse splicing, independent of homologous DNA recombination. Cell 94, 451–462 ( 1998).
Kuipers,O. P., Beerthuyzen,M. M., de Ruyter, P. G., Luesink,E. J. & de Vos,W. M. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J. Biol. Chem. 10, 27299–27304 ( 1995).
Biswas,I., Gruss,A., Ehrlich,S. D. & Maguin,E. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175, 3628–3625 (1993).
Mueller,M. W., Allmaier,M., Eskes,R. & Schweyen,R. J. Transposition of group II intron aI1 in yeast and invasion of mitochondrial genes at new locations. Nature 366, 174– 176 (1993).
Sellem,C. H., Lecellier,G. & Belcour, L. Transposition of a group II intron. Nature 366, 176–178 ( 1993).
Mohr,G., Smith,D., Belfort,M. & Lambowitz,A. M. Rules for DNA target site recognition by a Lactococcal intron enable retargeting of the intron to specific DNA sequences. Genes Dev. 14 , 559–573 (2000).
Roman,J. & Woodson,S. A. Reverse splicing of the Tetrahymena IVS: Evidence for multiple reaction sites in the 23S tRNA. RNA 1, 478–490 ( 1995).
Woodson,S. A. & Cech,T. R. Reverse self-splicing of the Tetrahymena group I intron: Implication for the directionality of splicing and for intron transposition. Cell 57, 335– 345 (1989).
Augustin,S., Muller,M. W. & Schweyen, R. J. Reverse self-splicing of group II intron RNAs in vitro. Nature 343, 383– 386 (1990).
Morl,M. & Schmelzer,C. Integration of group II intron bI1 into a foreign RNA by reversal of the self-splicing reaction in vitro. Cell 60, 629– 636 (1990).
Mohr,G. & Lambowitz,A. M. Integration of a group I intron into a ribosomal RNA sequence promoted by a tyrosyl-tRNA synthetase. Nature 354, 164–167 ( 1991).
Thompson,A. J. & Herrin,D. L. A chloroplast group I intron undergoes the first step of reverse splicing into host cytoplasmic 5.8 S rRNA. J. Mol. Biol. 236, 455– 468 (1994).
Roman,J., Rubin,M. N. & Woodson, S. A. Sequence specificity of in vivo reverse splicing of the Tetrahymena group I intron. RNA 5, 1–13 (1999).
Eickbush,T. H. Mobile introns: retrohoming by complete reverse splicing. Curr. Biol. 9, R11–R14 ( 1999).
Curcio,M. J. & Belfort,M. Retrohoming: cDNA-mediated mobility of group II introns requires a catalytic RNA. Cell 84, 9–12 (1996).
Ferat,J. & Michel,F. Group II self-splicing introns in bacteria. Nature 364, 358– 361 (1993).
Xiong,Y. & Eickbush,T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 9, 3353–3362 (1990).
Zimmerly,S., Guo,H., Perlman,P. S. & Lambowitz,A. M. Group II intron mobility occurs by target DNA-primed reverse transcription. Cell 82, 545–554 ( 1995).
Smit,A. F. The origin of interspersed repeats in the human genome. Curr. Opin. Genet. Dev. 6, 743–748 ( 1996).
Acknowledgements
We thank J. San Filippo and A. Lambowitz for providing the LtrA Endo- YRT mutant. We also thank D. Manias and G. Dunny for help in constructing pLEtd+KR″; D. Ehrlich and A. Sorokin for information based on the L. lactis DNA sequence; J. Curcio, K. Derbyshire, V. Derbyshire, S. Hanes, A. Lambowitz, R. Lease, R. Morse, D. Nag and M. Parker for comments on the manuscript; N. J. Schisler and J. Palmer for estimates of intron composition of the human genome; and M. Carl for manuscript preparation. This work was supported by NIH grants to M.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cousineau, B., Lawrence, S., Smith, D. et al. Retrotransposition of a bacterial group II intron. Nature 404, 1018–1021 (2000). https://doi.org/10.1038/35010029
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35010029
This article is cited by
-
Methylation of rRNA as a host defense against rampant group II intron retrotransposition
Mobile DNA (2021)
-
Transductomics: sequencing-based detection and analysis of transduced DNA in pure cultures and microbial communities
Microbiome (2020)
-
Recent horizontal transfer, functional adaptation and dissemination of a bacterial group II intron
BMC Evolutionary Biology (2016)
-
Crystal structures of a group II intron maturase reveal a missing link in spliceosome evolution
Nature Structural & Molecular Biology (2016)
-
RNA-Seq profile of flavescence dorée phytoplasma in grapevine
BMC Genomics (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.