Abstract
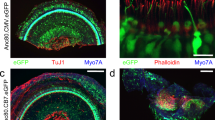
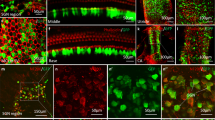
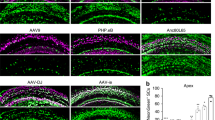
The confined fluid-filled labyrinth of the human inner ear presents an opportunity for introduction of gene therapy reagents designed to treat hearing and balance dysfunction. Here we present a novel model system derived from the sensory epithelia of human vestibular organs and show that the tissue can survive up to 5 days in vitro. We generated organotypic cultures from 26 human sensory epithelia excised at the time of labyrinthectomy for intractable Meniere's disease or vestibular schwannoma. We applied multiply deleted adenoviral vectors at titers between 105 and 108 viral particles/ml directly to the cultures for 4–24 h and examined the tissue 12–96 h post-transfection. We noted robust expression of the exogenous transgene, green fluorescent protein (GFP), in hair cells and supporting cells suggesting both were targets of adenoviral transfection. We also transfected cultures with a vector that carried the genes for GFP and KCNQ4, a potassium channel subunit that causes dominant-progressive hearing loss when mutated. We noted a positive correlation between GFP fluorescence and KCNQ4 immunolocalization. We conclude that our in vitro model system presents a novel and effective experimental paradigm for evaluation of gene therapy reagents designed to restore cellular function in patients who suffer from inner ear disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Petit C . From deafness genes to hearing mechanisms: harmony and counterpoint. Trends Mol Med 2006; 12: 57–64.
Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG et al. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol 1997; 137: 1287–1307.
Holt JR . Viral-mediated gene transfer to study the molecular physiology of the mammalian inner ear. Audiol Neurootol 2002; 7: 157–160.
Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004; 432: 723–730.
Luebke AE, Steiger JD, Hodges BL, Amalfitano A . A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther 2001; 8: 789–794.
Holt JR, Johns DC, Wang S, Chen ZY, Dunn RJ, Marban E et al. Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J Neurophysiol 1999; 81: 1881–1888.
Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 1999; 96: 437–446.
Oghalai JS, Holt JR, Nakagawa T, Jung TM, Coker NJ, Jenkins HA et al. Ionic currents and electromotility in inner ear hair cells from humans. J Neurophysiol 1998; 79: 2235–2239.
Holt JR, Corey DP . Ion channel defects in hereditary hearing loss. Neuron 1999; 22: 217–219.
Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA 2000; 97: 4333–4338.
Nair TS, Kozma KE, Hoefling NL, Kommareddi PK, Ueda Y, Gong TW et al. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. J Neurosci 2004; 24: 1772–1779.
Oghalai JS, Holt JR, Nakagawa T, Jung TM, Coker NJ, Jenkins HA et al. Harvesting human hair cells. Ann Otol Rhinol Laryngol 2000; 109: 9–16.
Lin D, Hegarty JL, Fischbein NJ, Jackler RK . The prevalence of “incidental” acoustic neuroma. Arch Otolaryngol Head Neck Surg 2005; 131: 241–244.
Howitz MF, Johansen C, Tos M, Charabi S, Olsen JH . Incidence of vestibular schwannoma in Denmark, 1977–1995. Am J Otol 2000; 21: 690–694.
Merchant SN, Adams JC, Nadol Jr JB . Pathophysiology of Meniere's syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol 2005; 26: 74–81.
Semaan MT, Alagramam KN, Megerian CA . The basic science of Meniere's disease and endolymphatic hydrops. Curr Opin Otolaryngol Head Neck Surg 2005; 13: 301–307.
Raphael Y, Frisancho JC, Roessler BJ . Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett 1996; 207: 137–141.
Han D, Yu Z, Fan E, Liu C, Liu S, Li Y et al. Morphology of auditory hair cells in guinea pig cochlea after transgene expression. Hear Res 2004; 190: 25–30.
Yamasoba T, Yagi M, Roessler BJ, Miller JM, Raphael Y . Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum Gene Ther 1999; 10: 769–774.
Stover T, Yagi M, Raphael Y . Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res 1999; 136: 124–130.
Stover T, Yagi M, Raphael Y . Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Therapy 2000; 7: 377–383.
Suzuki M, Yamasoba T, Kondo K, Kaga K . Transfection of young guinea pig vestibular cells in vitro with an adenovirus vector. Neuroreport 2001; 12: 4013–4017.
Yamasoba T, Suzuki M, Kondo K . Transgene expression in mature guinea pig cochlear cells in vitro. Neurosci Lett 2002; 335: 13–16.
Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y . Gene transfer into supporting cells of the organ of Corti. Hear Res 2002; 173: 187–197.
Staecker H, Li D, O'Malley BW, Van de Water TR . Gene expression in the mammalian cochlea: a study of multiple vector systems. Acta Otolaryngol 2001; 121: 157–163.
Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA et al. Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther 2001; 12: 539–548.
Kawamoto K, Oh SH, Kanzaki S, Brown N, Raphael Y . The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol Ther 2001; 4: 575–585.
Kanzaki S, Ogawa K, Camper SA, Raphael Y . Transgene expression in neonatal mouse inner ear explants mediated by first and advanced generation adenovirus vectors. Hear Res 2002; 169: 112–120.
Dazert S, Battaglia A, Ryan AF . Transfection of neonatal rat cochlear cells in vitro with an adenovirus vector. Int J Dev Neurosci 1997; 15: 595–600.
Dazert S, Aletsee C, Brors D, Gravel C, Sendtner M, Ryan A . In vivo adenoviral transduction of the neonatal rat cochlea and middle ear. Hear Res 2001; 151: 30–40.
Lalwani AK, Han JJ, Walsh BJ, Zolotukhin S, Muzyczka N, Mhatre AN . Green fluorescent protein as a reporter for gene transfer studies in the cochlea. Hear Res 1997; 114: 139–147.
Amalfitano A, Parks RJ . Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr Gene Ther 2002; 2: 111–133.
Amalfitano A . Utilization of adenovirus vectors for multiple gene transfer applications. Methods 2004; 33: 173–178.
Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y . Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci 2003; 23: 4395–4400.
Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med 2005; 11: 271–276.
Boeda B, Weil D, Petit C . A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum Mol Genet 2001; 10: 1581–1589.
Baum SG . Adenovirus.In: Mandell GL, Bennet JE, Dolin R, (eds). Principles and Practice of Infectious Diseases, 6th edn. Elesevier, Inc.: Philadelphia, 2005, pp 1835–1841.
Venail F, Wang J, Ruel J, Ballana E, Rebillard G, Eybalin M et al. Coxsackie adenovirus receptor and alphanubeta3/alphanubeta5 integrins in adenovirus gene transfer of rat cochlea. Gene Therapy 2007; 14: 30–37.
Derby ML, Sena-Esteves M, Breakefield XO, Corey DP . Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear Res 1999; 134: 1–8.
Han JJ, Mhatre AN, Wareing M, Pettis R, Gao WQ, Zufferey RN et al. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum Gene Ther 1999; 10: 1867–1873.
Stone IM, Lurie DI, Kelley MW, Poulsen DJ . Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther 2005; 11: 843–848.
Lalwani AK, Jero J, Mhatre AN . Current issues in cochlear gene transfer. Audiol Neurootol 2002; 7: 146–151.
Lalwani AK, Mhatre AN . Cochlear gene therapy. Ear Hear 2003; 24: 342–348.
Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD et al. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci 2002; 5: 41–47.
Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R et al. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J 2006; 25: 642–652.
Staecker H, Praetorius M, Baker K, Brough DE . Vestibular hair cell regeneration and restoration of balance function induced by Math1 gene transfer. Otol Neurotol 2007; 28: 223–231.
Johns DC, Marx R, Mains RE, O'Rourke B, Marban E . Inducible genetic suppression of neuronal excitability. J Neurosci 1999; 19: 1691–1697.
Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS . Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol 1998; 72: 926–933.
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B . A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Hodges BL, Serra D, Hu H, Begy CA, Chamberlain JS, Amalfitano A . Multiply deleted [E1, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Med 2000; 2: 250–259.
Acknowledgements
We thank Dr Thomas Jentsch and Dr Kirk Biesel for providing the coding sequence of the human and mouse KCNQ4 genes, respectively. We also thank Dr Andrea Amalfitano for providing Ad2-GFP, Dr Bechara Kachar for the KCNQ4 antibody and Dr Tama Hasson for the myosin-VIIa antibody. This work was supported by the NIH/NIDCD Grant #DC005439 to JR Holt, the Virginia Lion's Hearing Foundation and a Triological Society Career Development Grant to BW Kesser.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Gene Therapy Web site (http://www.nature.com/gt)
Supplementary information
Rights and permissions
About this article
Cite this article
Kesser, B., Hashisaki, G., Fletcher, K. et al. An in vitro model system to study gene therapy in the human inner ear. Gene Ther 14, 1121–1131 (2007). https://doi.org/10.1038/sj.gt.3302980
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302980
Keywords
This article is cited by
-
A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear
Nature Biotechnology (2017)
-
Emerging Gene Therapies for Genetic Hearing Loss
Journal of the Association for Research in Otolaryngology (2017)
-
Adeno-associated virus-mediated gene transfer targeting normal and traumatized mouse utricle
Gene Therapy (2014)
-
Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear
Gene Therapy (2011)