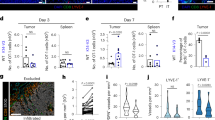
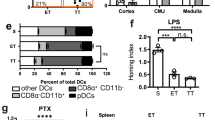
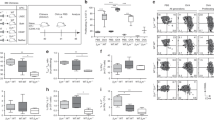
Abstract
Peripheral lymph nodes (PLN) are strategic microenvironments where antigen-presenting dendritic cells (DC), loaded with environmental antigens, and naive lymphocytes meet to initiate immune responses. The unique capacity of DC to induce primary immune responses has led to their use in clinical medicine; however, delivering DC to lymph nodes is problematic. Intravenously injected DC cannot access to PLN, while DC injected into tissue migrate inefficiently through lymphatics to PLN. We achieved DC targeting to T-cell areas of PLN by endowing DC with a novel receptor for peripheral node addressin (PNAd), an adhesion molecule present on the lymph node venular endothelium. This novel receptor is a chimeric E/L-selectin (ELS) that, we have previously shown, binds to PNAd. DC were genetically modified by retroviral transduction to express ELS. ELS expression was targeted to tips of microvilli, and mediated rolling of DC on PNAd both in vivo and in vitro. Such genetically engineered DC could extravasate directly from blood through the lymph node endothelium as opposed to nontransduced DC. This study provides evidence that the trafficking of DC can be modified using gene transfer technologies. More efficient delivery of DC to PLN should assist the development of improved vaccination strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Caux C et al. Regulation of dendritic cell recruitment by chemokines. Transplantation 2002; 73: S7–S11.
von Andrian UH, Mackay CR . T-cell function and migration. Two sides of the same coin. N Engl J Med 2000; 343: 1020–1034.
Sallusto F et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol 1999; 29: 1617–1625.
Kunkel EJ, Butcher EC . Chemokines and the tissue-specific migration of lymphocytes. Immunity 2002; 16: 1–4.
Butcher EC, Picker LJ . Lymphocyte homing and homeostasis. Science 1996; 272: 60–66.
Weninger W, Crowley MA, Manjunath N, von Andrian UH . Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med 2001; 194: 953–966.
Morse MA et al. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res 1999; 59: 56–58.
Eggert AA et al. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res 1999; 59: 3340–3345.
Lappin MB et al. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology 1999; 98: 181–188.
Stein JV et al. L-selectin-mediated leukocyte adhesion in vivo: microvillous distribution determines tethering efficiency, but not rolling velocity. J Exp Med 1999; 189: 37–50.
Nestle FO et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4: 328–332.
Kansas GS . Selectins and their ligands: current concepts and controversies. Blood 1996; 88: 3259–3287.
von Andrian UH et al. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell 1995; 82: 989–999.
Warnock RA, Askari S, Butcher EC, von Andrian UH . Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med 1998; 187: 205–216.
Kahn J, Ingraham RH, Shirley F, Migaki GI, Kishimoto TK . Membrane proximal cleavage of L-selectin: identification of the cleavage site and a 6-kD transmembrane peptide fragment of L-selectin. J Cell Biol 1994; 125: 461–470.
Lawrence MB, Springer TA . Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 1991; 65: 859–873.
Ley K, Allietta M, Bullard DC, Morgan S . Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res 1998; 83: 287–294.
Kunkel EJ, Ley K . Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res 1996; 79: 1196–1204.
von Andrian UH . Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 1996; 3: 287–300.
Stockton BM, Cheng G, Manjunath N, Ardman B, von Andrian UH . Negative regulation of T cell homing by CD43. Immunity 1998; 8: 373–381.
Robert C, Kupper TS . Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med 1999; 341: 1817–1828.
Campbell DJ, Butcher EC . Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med 2002; 195: 135–141.
Thurner B et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 1999; 190: 1669–1678.
Banchereau J et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 2001; 61: 6451–6458.
Robert C et al. Interaction of dendritic cells with skin endothelium: a new perspective on immunosurveillance. J Exp Med 1999; 189: 627–636.
Pickl WF et al. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol 1996; 157: 3850–3859.
Klein C, Bueler H, Mulligan RC . Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med 2000; 191: 1699–1708.
Berg EL, Robinson MK, Warnock RA, Butcher EC . The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol 1991; 114: 343–349.
De Clerck LS, Bridts CH, Mertens AM, Moens MM, Stevens WJ . Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. J Immunol Methods 1994; 172: 115–124.
von Andrian UH, M'Rini C . In situ analysis of lymphocyte migration to lymph nodes. Cell Adhes Commun 1998; 6: 85–96.
Acknowledgements
Caroline Robert was supported by a Dermatology Foundation fellowship. This work was supported by National Institutes of Health grants R01 HL54936, R01 HL62524, P01 HL 56949 (to UH von Andrian) and R37 AI25082, R01 AI41707, R01 AR40124, P30 AR42689 (to TS Kupper). Christoph Klein was initially supported by the Deutsche Forschungsgemeinschaft and is a Scholar of the American Society of Hematology.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robert, C., Klein, C., Cheng, G. et al. Gene therapy to target dendritic cells from blood to lymph nodes. Gene Ther 10, 1479–1486 (2003). https://doi.org/10.1038/sj.gt.3302008
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302008
Keywords
This article is cited by
-
Induction of protective cytotoxic T-cell responses by a B-cell-based cellular vaccine requires stable expression of antigen
Gene Therapy (2009)
-
Introduction of functional chimeric E/L-selectin by RNA electroporation to target dendritic cells from blood to lymph nodes
Cancer Immunology, Immunotherapy (2008)
-
Activation of bone marrow–resident memory T cells by circulating, antigen-bearing dendritic cells
Nature Immunology (2005)
-
Immune surveillance in the skin: mechanisms and clinical consequences
Nature Reviews Immunology (2004)
-
Homing and cellular traffic in lymph nodes
Nature Reviews Immunology (2003)