Abstract
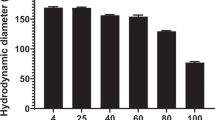
Nucleic acids delivery vectors have shown promising therapeutic potential in model systems. However, comparable clinical success is delayed essentially because of their poor biodistribution and of their ineffective intracellular trafficking. The size of condensed DNA particles is a key determinant for in vivo diffusion, as well as for gene delivery to the cell nucleus. Towards this goal, we have developed cationic thiol-detergents that individually compact plasmid DNA molecules into anionic particles. These particles are then ‘stabilized’ by air-induced dimerization of the detergent into a disulfide lipid on the template DNA. The particles all measure approximately 30 nm, which corresponds to the volume of a single molecule of plasmid DNA. The gel electrophoretic mobility of the anionic particles was found to be higher than that of the plasmid DNA itself. Similarly, particles formed with a 31-mer oligonucleotide measured 19 nm. Improved in vivo diffusion, as well as improved intracellular trafficking may be inferred from the faster migration of the complexes. Moreover, the size of the particles remains compatible with nuclear pore crossing. Finally, in an attempt to improve the biodistribution of these particles, we have coated the monomolecular particles with a poly(ethylene glycol) corona.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lukacs GL et al. Size-dependent DNA mobility in cytoplasm and nucleus J Biol Chem 2000 275: 1625–1629
Feldherr CM, Akin D . Signal-mediated nuclear transport in proliferating and growth-arrested BALB/c 3T3 cells J Cell Biol 1991 115: 933–939
Tang MX, Szoka FC . The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes Gene Therapy 1997 4: 823–832
Behr JP . DNA strongly binds to micelles and vesicles containing lipopolyamines or lipointercalants Tetrahedron Lett 1986 27: 5861–5864
Mel'nikov SM, Sergeyev VG, Yoshikawa K . Discrete coil-globule transition of large DNA induced by cationic surfactant J Am Chem Soc 1995 117: 2401–2408
Remy JS, Sirlin C, Vierling P, Behr JP . Gene transfer with a series of lipophilic DNA-binding molecules Bioconj Chem 1994 5: 647–654
Clamme JP et al. Gene transfer by cationic surfactants is essentially limited by the trapping of the surfactant/DNA complexes onto the cell membrane: a fluorescence investigation Biochim Biophys Acta 2000 1467: 347–361
Blessing T, Remy J-S, Behr J-P . Template oligomerization of DNA-bound cations produces calibrated nanometric particles J Am Chem Soc 1998 120: 8519–8520
Blessing T, Remy JS, Behr JP . Monomolecular collapse of plasmid DNA into stable virus-like particles Proc Natl Acad Sci USA 1998 95: 1427–1431
Dauty E, Remy JS, Blessing T, Behr JP . Dimerizable cationic detergents with a low cmc condense plasmid DNA into nanometric particles and transfect cells in culture J Am Chem Soc 2001 123: 9227–9234
Labat-Moleur F et al. An electron microscopy study into the mechanism of gene transfer with lipopolyamines Gene Therapy 1996 3: 1010–1017
Mislick KA, Baldeschwieler JD . Evidence for the role of proteoglycans in cation-mediated gene transfer Proc Natl Acad Sci USA 1996 93: 12349–12354
Behr JP, Demeneix B, Loeffler JP, Perez-Mutul J . Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA Proc Natl Acad Sci USA 1989 86: 6982–6986
Boussif O, Zanta MA, Behr JP . Optimized galenics improve in vitro gene transfer with cationic molecules up to thousand-fold Gene Therapy 1996 3: 1074–1080
Woodle MC, Lasic DD . Sterically stabilized liposomes Biochim Biophys Acta 1992 1113: 171–199
Allen TM et al. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo Biochim Biophys Acta 1991 1066: 29–36
Plank C, Mechtler K, Szoka FC Jr., Wagner E . Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery Hum Gene Ther 1996 7: 1437–1446
Ogris M et al. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery Gene Therapy 1999 6: 595–605
Zanta MA, Boussif O, Adib A, Behr JP . In vitro gene delivery to hepatocytes with galactosylated polyethylenimine Bioconj Chem 1997 8: 839–844
Acknowledgements
This work was supported by Vaincre la Mucoviscidose.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dauty, E., Behr, JP. & Remy, JS. Development of plasmid and oligonucleotide nanometric particles. Gene Ther 9, 743–748 (2002). https://doi.org/10.1038/sj.gt.3301759
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301759
Keywords
This article is cited by
-
Nanosized bioceramic particles could function as efficient gene delivery vehicles with target specificity for the spleen
Gene Therapy (2007)
-
Artificial viruses: a nanotechnological approach to gene delivery
Nature Reviews Drug Discovery (2006)