Abstract
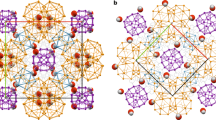
Chemical self-assembly is the process by which ‘programmed’ molecular subunits spontaneously form complex supramolecular frameworks1,2. This approach has been applied to many model systems, in which hydrogen bonds3,4, metal–ligand coordination5 or other non-covalent interactions6 typically control the self-assembly process. In biology, self-assembly is generally dynamic and depends on the cooperation of many such non-covalent interactions. Water can play an important role in these biological self-assembly processes, for example by stabilizing the native conformation of biopolymers7,8,9. Hydrogen-bonded (H2O)n clusters10,11 can play an important role in stabilizing some supramolecular species, both natural and synthetic, in aqueous solution. Here we report the preparation and crystal structure of a self-assembled, three-dimensional supramolecular complex that is stabilized by an intricate array of non-covalent interactions involving contributions from solvent water clusters, most notably a water decamer ((H2O)10) with an ice-like molecular arrangement. These findings show that the degree of structuring that can be imposed on water by its surroundings, and vice versa, can be profound.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives (VCH, Weinheim, (1995)).
Lehn, J.-M. Supramolecular chemistry. Science 260, 1762–1763 (1993).
Seto, C. T. & Whitesides, G. M. Molecular self-assembly through hydrogen bonding: Supramolecular aggregates based on the cyanuric acid · melamine lattice. J. Am. Chem. Soc. 115, 905–916 (1993).
Wyler, R., de Mendoza, J. & Rebek, J. Asynthetic cavity assembles through self-complementary hydrogen bonds. Angew. Chem. Int. Edn Engl. 32, 1699–1701 (1993).
Hasenknopf, B., Lehn, J.-M., Kneisel, B., Baum, O. G. & Fenske, D. Self-assembly of a circular double helicate. Angew. Chem. Int. Edn Engl. 35, 1838–1840 (1996).
Oriol, J. et al. Adesigned non-peptidic receptor that mimics the phosphocholine binding site of the McPC603 antibody. Angew. Chem. Int. Edn Engl. 35, 1712–1715 (1996).
Dehl, R. E. & Hoeve, C. A. Broad-line NMR study of H2O and D2O in collagen fibres. J. Chem. Phys. 50, 3245–3251 (1969).
Migchelsen, C., Berendsen, H. J. C. & Rupprecht, A. Hydration of DNA. Comparison of nuclear magnetic resonance results for oriented DNA in the A, B and C form. J. Mol. Biol. 37, 235–237 (1968).
Steckel, F. & Szapiro, S. Physical properties of heavy oxygen water. Part I–Density and thermal expansion. Trans. Faraday Soc. 59, 331–343 (1963).
Colson, S. D. & Dunning, T. H. The structure of Nature's solvent: water. Science 265, 43–44 (1994).
Liu, K., Cruzan, J. D. & Saykally, R. J. Water clusters. Science 271, 929–933 (1996).
König, H. Acubic ice modification. Z. Kristallogr. 105, 279–286 (1944).
Eisenberg, D. & Kauzmann, W. The Structure and Properties of Water (Oxford Univ. Press, New York, (1969)).
Franks, F. (ed.) Water: A Comprehensive Treatise Vols 1–4;(Plenum, New York, (1972)).
Westhoff, E. (ed.) Water and Biological Macromolecules (CRC Press, Boca Raton, (1993)).
Jeffrey, G. A. in Inclusion Compounds Vol. 1(eds Atwood, J. L., Davies, J. E. D. & MacNicol, D. D.) Ch. 5 (Academic, London, (1984)).
Park, K.-M., Kurodo, R. & Iwamoto, T. Atwo-dimensional ice with the topography of edge-sharing hexagons intercalated between CdNi(CN)4layers. Angew. Chem. Int. Edn Engl. 32, 884–886 (1993).
Rau, D. C. & Parsegian, V. A. Direct measurement of forces between linear polysaccharides Xanthan and Schizophyllan. Science 249, 1278–1281 (1990).
Royer, W. E., Pardanani, A., Gibson, Q. H., Peterson, E. S. & Feldman, J. M. Ordered water molecules as key allosteric mediators in a cooperative dimeric hemoglobin. Proc. Natl Acad. Sci. USA 93, 14526–14531 (1996).
Teeter, M. M. Water structure of a hydrophobic protein at atomic resolution: Pentagon rings of water molecules in crystals of crambin. Proc. Natl Acad. Sci. USA 81, 6014–6018 (1984).
Baker, E. N. Structure of actinidin, after refinement at 1.7 Å resoution. J. Mol. Biol. 141, 441–484 (1980).
Liljas, A. et al. Crystal structure of human carbonic anhydrase C. Nature New Biol. 235, 131–137 (1972).
Quiocho, F. A., Wilson, D. K. & Vyas, N. K. Substrate specificity and affinity of a protein modulated by bound water molecules. Nature 340, 404–407 (1989).
Acknowledgements
We thank C. Barnes for assistance with the unit cell and space group determination. This work was supported by the US NSF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbour, L., Orr, G. & Atwood, J. An intermolecular (H2O)10 cluster in a solid-state supramolecular complex. Nature 393, 671–673 (1998). https://doi.org/10.1038/31441
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/31441
This article is cited by
-
Synthesis, Crystal Structure and Thermal Property of Two New Coordination Polymers with Ligands Containing Both N and O Donors
Journal of Chemical Crystallography (2020)
-
Predominantly ligand guided non-covalently linked assemblies of inorganic complexes and guest inclusions
Journal of Chemical Sciences (2018)
-
Simultaneous assembly of mononuclear and dinuclear dysprosium(III) complexes behaving as single-molecule magnets in a one-pot hydrothermal synthesis
Science China Chemistry (2017)
-
Hybrid Cyclic Water–Chloride Cluster Self-assembled in a Ruthenium(II) Polypyridyl Complex
Journal of Chemical Crystallography (2016)
-
Synthesis, Structures and Properties of Two New Coordination Polymers with Unprecedented Water Cluster
Journal of Cluster Science (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.