Abstract
Study design:
To compare results obtained with a variety of locomotor rating scales in Th9/10 spinal cord transected (Tx) mice.
Objectives:
To assess spontaneous recovery with a variety of rating scales to find the most sensitive methods for assessing recovery levels in Tx mice and differences associated with gender and condition.
Setting:
Laval University Medical Center, Neuroscience Unit & Laval University, Department of Anatomy and Physiology, Quebec City, Quebec, Canada.
Methods:
Scales including the Basso, Beattie and Bresnahan (BBB), the Basso Mouse Score (BMS), the Antri, Orsal and Barthe (AOB), the Motor Function Score (MFS) and the Averaged Combined Score (ACOS) were used to assess, in open-field and treadmill conditions, spontaneous locomotor recovery in male and female Tx mice.
Results:
The ACOS scale revealed a progressive increase of spontaneous recovery during 5-weeks post-Tx. The other methods detected a progressive increase for the first 2–3 weeks post-Tx without any significant progress in weeks 4 and 5. Generally, scores obtained with each method were nonsignificantly different between males and females or between open-field and treadmill conditions.
Conclusion:
These results further confirm the existence of a limited but significant increase of locomotor function recovery, occurring without intervention, in Tx animals. Although each method could detect small levels of recovery, the ACOS method was discriminative enough to detect progressive changes up to 5 weeks post-Tx. In conclusion, the ACOS rating scale was the most discriminative method for assessing the spontaneous return of hindlimb movements found in Tx mice, both in open-field and treadmill conditions.
Similar content being viewed by others
Introduction
Animal models have been increasingly used in the last 20 years to investigate the pathological changes induced by spinal cord injury (SCI). These models have allowed the study of potential new treatments and approaches to reduce secondary cellular damage and scar formation or to increase neuronal regeneration and reconnection.1 Recently, mice have been used more frequently for SCI research due to the availability of genetically engineered models and molecular tools.2 Murine models with different types of SCI such as contusion, displacement, crush, clip compression, ischemia and transection are commonly used for investigations.3
A number of scales and methods are available to assess functional recovery levels in SCI mice. One of the most commonly used methods is the Basso, Beattie and Bresnahan locomotor rating scale (BBB).4 However, this scale has been designed specifically for spinal cord contused rats in open-field conditions and its utilization in mice has been reported as problematic.5, 6 Consequently, efforts have been made to develop alternative methods adapted to SCI mice – adapted BBB,5 Motor Function Score (MFS),7 Basso Mouse Scale (BMS),8 and Average Combined Score (ACOS).9 These methods take into account the fact that the hindlimb main articulations (hip, knee and ankle) are not all easily detectable in SCI mice9 that progression of locomotor function recovery, is different in mice than in rats (eg progression of tail movements)6 and that mice do not exhibit visually detectable differences in toe drag.5
In addition, concerns have been raised by some researchers that most currently used methods are not appropriate or sensitive enough to evaluate severely SCI or Tx animals.9, 10 For instance, forelimb versus hindlimb coordination or fine foot placement, assessed by most standard methods, constitutes irrelevant criteria for evaluation of Tx animals. In line with this, most studies with Tx mice produced recovery levels that are considered nonsignificant by their authors.7, 11, 12 However, it has been clearly shown, using alternative methods, that some significant levels of spontaneous motor and locomotor recovery can be found in completely spinal cord transected (Tx) mammals. Indeed, average scores up to level 5 have been reported after 1 month with the 22 level-AOB (Antri, Orsal and Barthe) scale in untreated spinalized rats.13 Spontaneous full weight-bearing steps at relatively low treadmill speeds have been described in untrained but tail-stimulated spinal cats after 2–3 weeks post-Tx.14 Also, in spinal mice, weight-bearing steps and plantar foot placements have been detected in a few cases with tests performed on a motor-driven treadmill at relatively low-speeds with tail stimulation.15 Without intervention (ie no graft, drug treatment, tail stimulation or body-weight support), small but significant levels of spontaneous recovery have been reported in spinal mice. Indeed, rhythmic bilaterally alternated movements of small amplitude (ie locomotor-like but with no weight-bearing and plantar foot placement capabilities) have been found after 2–3 weeks in the hindlimbs of Tx mice tested in open-field conditions.9 Taken together, these results have demonstrated the existence of spontaneous locomotor function recovery in completely spinalized and developmentally mature mammals. However, locomotor scales have never been compared to determine, in completely spinalized mice, which better detect this type of recovery.
Here, we examined a number of locomotor scales to assess spontaneous locomotor recovery levels in the hindlimbs of low-thoracic Tx mice. Differences between males and females, as well as between open-field and treadmill conditions, were also examined. The aim was to identify which of these methods are better suited to assess spontaneous recovery in completely spinalized mice.
Methods
Animal model and surgical procedures
All experimental procedures were conducted in accordance with the Canadian Council for Animal Care guidelines and accepted by the Laval University Animal Care and Use Committee. A total of 22 mice (11 male and 11 female CD1 mice, Charles River Canada, St-Constant, Quebec, Canada), approximately 8-week-old and initially weighing 30–40 g were used for this study. All mice were spinal cord Tx at the low-thoracic level.16, 17, 18 In brief, preoperative care included subcutaneous injection of 1 ml lactate-Ringer's solution, an analgesic (0.1 mg/kg buprenorphine) and an antibiotic (5 mg/kg Baytril). A complete transection of the spinal cord was performed intervertebrally using microscissors inserted between the 9th and 10th thoracic vertebrae in mice under complete anesthesia with 2.5% isoflurane. To ensure that complete transection was achieved, the inner vertebral walls were explored and entirely scraped several times with scissor tips in order to disrupt any small fibers which had not been severed. The incision was then sutured and the animals were placed on a heating pad for a few hours. Postoperative care, provided for four days, included subcutaneous injection of lactate-Ringer's solution (2 × 1 ml/day), buprenorphine (0.2 mg/kg/day) and Baytril (5 mg/kg/day). Bladders were emptied manually until a spontaneous return of the micturition reflex. Animals were left in their cage with food and water ad libitum. Complete spinal cord transection was confirmed by (1) initial full paralysis of the hindlimbs, (2) post-mortem visual examination of the spinal cord lesion for evidence of spared tissue and (3) coronal or midsagittal spinal cord sections stained with luxol fast blue/cresyl violet for myelinated descending axons and Nissl substance.
Experimental protocol and assessment methods
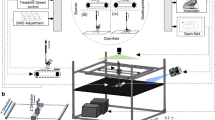
Mice were left resting in their cage for two days after surgery to allow recovery before testing. Tests were performed at 3, 7, 14, 21, 28 and 35 days post-Tx in both open-field and treadmill conditions in a randomized manner to avoid carried-over fatigue. Animals were also allowed to rest for approximately 40 min between the two conditions. Tests at 3 days were considered as a control, given that essentially no sign of hindlimb movement recovery is observed after only few days post-Tx.9 In open-field conditions, mice were examined inside a closed circular arena (60 × 60 cm) entirely made of transparent plexiglas to facilitate video camera monitoring and recording.9 In treadmill conditions, we used a custom-made 10-track adjustable-speed treadmill running at 8–10 cm/s.17, 18, 19 Mice were filmed using a digital video camera system (Sony DCR-PC9, shutter speed: 1/1000; acquisition: 30 frames/s) fixed on a tripod and positioned at a 45° angle above (open-field) or behind (treadmill) in order to observe most hindlimb movement characteristics including bilateral alternation. Data were directly collected and stored on a computer, before being displayed and analyzed off-line by two trained observers. Five different methods for assessing locomotor recovery were chosen based on their complementarity. Although some of these methods have not necessarily been designed for spinal cord Tx mice to be tested both in open-field and treadmill conditions, they have been shown to provide, in some cases, valuable information in both conditions.10, 18 As many of these methods have already been used in very different conditions than originally designed for (eg BBB used in mice, see Engesser-Cesar et al;8 Ma et al12), it became of interest to compare them, in the same study, in order to clearly establish whether or not some methods are more sensitive than others for Tx mice.
Basso, Beattie and Bresnahan locomotor scale
This locomotor rating scale4, 20 has been used extensively for the last 10 years to assess locomotor performance in incompletely spinal cord injured rats. It consists of 21 discriminative levels with progressively increasing scores:
-
0 – No hindlimb movement.
-
1 – Slight movement of one or two joints, usually the hip and/or the knee.
-
2 – Extensive movement of one joint with or without slight movement of one other joint.
-
3 – Extensive movement of two joints.
-
4 – Slight movement of all three joints.
-
5 – Slight movement of two joints and extensive movement of the third.
-
6 – Extensive movement of two joints and slight movement of the third.
-
7 – Extensive movement of all three joints.
All scores above 7 include some additional levels of plantar foot placement and/or weight support (for details, see Basso et al20). Slight or extensive amplitude were defined as less than half or more than half the normal range of joint motion, respectively. Occasional, frequent and consistent were defined as <50, 51–94 and >95% of total number of observed movements. Scores were determined for each of the two hindlimbs and then averaged. Note that a modified BBB scale has been developed recently by Dergham et al.5 However, only scoring levels in the upper range of the scale were modified, which is why we did not use it for testing in addition to the BBB scale.
Antri, Orsal and Barthe motor scale
This locomotor rating scale created by Antri et al,10 was designed specifically to assess hindlimb movements in Tx rodents. It does not assess forelimb versus hindlimb coordination, which is considered irrelevant in assessing spontaneous recovery in Tx animals. This is because regenerative processes across the lesion in adults are only possible in animals with partially injured spinal cords, following grafting interventions, or through the use of specific regenerative treatments. The AOB scale consists of 22 discriminative scores:
-
0 – No movement.
-
1 – Weak limb jerks.
-
2 – Weak rhythmic movements with no bilateral alternation.
-
3 – Large rhythmic movements with no bilateral alternation.
-
4 – Weak rhythmic movements with occasional bilateral alternation.
-
5 – Large rhythmic movements with occasional bilateral alternation.
-
6 – Weak rhythmic movements with frequent bilateral alternation.
-
7 – Large rhythmic movements with frequent bilateral alternation.
-
8 – Weak rhythmic movements with consistent bilateral alternation.
-
9 – Large rhythmic movements with consistent bilateral alternation.
Additional levels include body-weight support and plantar foot placement capabilities (for additional details, see Antri et al10). Conditions of observation and criteria for evaluating amplitude and frequency were the same as for the BBB scale (see above). Note that characteristics occurring only rarely (eg, once or twice) were not considered sufficient to fulfill the requirements for up-grades to higher corresponding levels.
Basso Mouse Scale
This method was developed for incomplete SCI mice preferably in open-field conditions. It takes into account that locomotor recovery progression is different in mice than in rats.8 It is a 9-point scale divided as follows:
-
0 – No ankle movement.
-
1 – Slight ankle movement.
-
2 – Extensive ankle movement.
-
3 – Plantar placing of the paw with or without weight support or occasional, frequent or consistent dorsal stepping but no plantar stepping.
-
4 – Occasional plantar stepping.
-
5 – Frequent or consistent plantar stepping, no coordination or frequent or consistent plantar stepping, some coordination, paws rotated at initial contact and lift off.
-
6 – Frequent or consistent plantar stepping, some coordination, paws parallel at initial contact or frequent or consistent plantar stepping, mostly coordinated, paws rotated at initial contact and lift off.
-
7 – Frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and rotated at lift off or frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and lift off, and severe trunk instability.
-
8 – Frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and lift off and mild trunk instability or frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and lift off, normal trunk stability and tail down or up and down.
-
9 – Frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and lift off, normal trunk stability and tail always up.
Hindlimb Motor Function Score (MFS)
This method was developed by Farooque7 specifically to evaluate SCI mice. It consists of a 10-point scale:
-
0 – No movement of the hindlimbs.
-
1 – Barely perceptible movement of any hindlimb joints (hip, knee or ankle).
-
2 – Brisk movements at one or more hindlimb joints in one or both limbs but no coordination.
-
3 – Alternate stepping and propulsive movements of hindlimbs but no weight bearing.
-
4 – Weight bearing and can walk with some deficit.
-
5 – Normal walking.
-
6 – Normal walking and can walk on a 2-cm-wide bar.
-
7 – Can walk on a 1.5-cm-wide-bar.
-
8 – Can walk on a 1-cm-wide-bar.
-
9 – Can walk on a 0.7-cm-wide-bar.
-
10 – Can walk on a 0.5-cm-wide-bar.
Average Combined Score
This method is used routinely in our laboratory.9, 17, 18 In addition to being partially quantitative and therefore more objective, it is a useful method for distinguishing locomotor-like movements (LM) from nonlocomotor movements (NLM) in the hindlimbs of spinalized mice. LM and NLM frequency, incidence and amplitude were assessed during a 4-min bout of video-recorded activity.9 To ease comparisons, a unique score ACOS is created by simple arithmetic combination of the collected values – NLM and LM frequency (per min), amplitude and incidence (see below for details, ACOS=[NLM+(2 × LM)] × amplitude). One LM was defined as an entire step-like cycle consisting of an extension phase or stance followed by a flexion phase or swing occurring in both hindlimbs consecutively (ie bilaterally alternated or out-of-phase relation). Extension began with foot contact onset (ie dorsal or plantar foot) until the lift off or the end of foot contact with the ground or treadmill belt. Flexion began with foot contact ending (ie lift off) until next foot contact or extension onset. In the case where the foot never quite cleared the ground or was constantly rubbing against the treadmill belt (or the ground), then extension was more generally defined as when the hindlimb was in a relatively extended position, and flexion when it was not extended and generally flexed. One NLM was defined as one nonbilaterally coordinated movement (ie not followed by a flexion-extension on the other side). They included unilateral movements, jerks, brief sequences of fast-paw shaking (typically lasting 1–2 s/episode and counted as one NLM), twitches and kicks. Amplitude was characterized by assigning one of three values; (0) if no movement was observed; (1) if the amplitude of most movements was less than half the range of motion of normal steps; (2) if the amplitude of most movements was at least more than half the range of motion of normal steps. Note that amplitude was scored for LM and NLM indistinctively. Incidence corresponded with the number of mice (out of all mice tested in a group) in which NLMs or LMs were observed. Plantar foot placement and body-weight support were reported as either present or not. Note that, in the equation, LM is multiplied by a factor of ‘2’ for very simple and logical reasons. We consider, from our experience, that it is easier for most observers to count as ‘1’ rather than ‘2’, an event defined as one bilaterally alternating movement (ie 1 LM). However, what are being described really are two consecutive movements (one in each of the two hindlimbs). To respect the ‘linearity’ of score progression of the ACOS method, then one LM (one movement in each of the two hindlimbs) which is then twice more ‘valuable’ than only one NLM (one single hindlimb movement) should therefore, in the end, be multiplied by a factor of ‘2’ to reflect the fact that two movements are really being described with one LM.
Statistics
Results were reported as means±SE. For differences between days, a Friedman test followed by a Dunn's Multiple Comparison test was used. In order to evaluate gender- and condition-related differences, a two-way repeated measure ANOVA followed by a Bonferonni post hoc was used. Statistical differences between the linear regression slopes were examined with ANCOVA. P-values <0.05 were considered statistically significant.
Results
Low-thoracic spinal cord Tx mice (males and females) were filmed over a period of 4 min in open-field and in treadmill conditions at 3, 7, 14, 21, 28 and 35 days postsurgery. Spontaneously occurring hindlimb movements were subsequently analyzed (off-line) using five different locomotor scoring methods. Data from male and female mice were generally pooled together except for Figure 4 where gender-related differences were specifically examined.
Recovery levels with time
Figure 1a shows that Tx mice developed spontaneous hindlimb movement under both conditions that corresponded to average scores lower than level 1 on the BBB scale during the first 2 weeks post-Tx. Indeed, increasing scores, although considered as nonsignificant (P=0.066), were found at 7 days (0.40±0.07) compared with controls (3 days, 0.16±0.06). At 14 and 21 days, further increased scores that reached 0.84±0.09, (P<0.01) and 1.40±0.16, (P<0.001), respectively, were found to be significantly greater than controls (Figure 1a). No significant additional progress (P>0.05) was detected between 21, 28 and 35 days showing that performances assessed with the BBB scale reached a plateau level at 21 days (1.40±0.15) until the end of the study period (1.62±0.18 at 35 days).
Spontaneous recovery assessed with qualitative methods. Evaluation of spontaneous recovery with four different qualitative scales (a) BBB, (b) AOB, (c) BMS and (d) MFS. Three days after spinal cord transection, score levels close to zero. This time point served as a control. For each method tested, a nonsignificant increase was observed at 7 days. Compared with day 3, significant levels of recovery were found at 14 days and subsequently. After 21 days, all scales reached a plateau as no significant differences were found between scores at 21, 28 and 35 days. *P<0.01, **P<0.001. Data were pooled from open-field and treadmill conditions
Comparable results were found with the AOB scale. Indeed, Figure 1b shows increasing average scores that reached 0.52±0.08 and 0.93±0.09 at 7 and 14 days post-Tx, respectively. Significant differences (P<0.001) compared with controls (0.20±0.09 at 3 days) were found at 14, 21, 28 and 35 days (Figure 1b).
As with the BBB scale, performances assessed with the AOB scale reached a plateau at 21 days after which no additional significant progress (P>0.05) was found when comparing 21–28 days and 28–35 days.
The BMS scale evaluated recovery levels to be comparable with those described above. An increase, although nonsignificant (P=0.059), was found at 7 days post-Tx (0.38±0.08) compared with controls (0.14±0.07, Figure 1c). Further significant increases (P<0.001) were observed until a plateau level was reached at 14 days post-Tx. Similar results were found with the MFS scale with significant (P<0.001) increases in performance post-Tx (ie at 14 days and later) until a plateau level was reached at 21 days (Figure 1d).
The ACOS method detected a progressive increase in performance up to 35 days post-Tx (Figure 2e). At 7 days, an increase (0.66±0.18) close to the level of significance (P=0.051) was found compared with controls (0.09±0.04). Further significantly (P<0.001) higher scores were found at 14, 21, 28 and 35 days. In contrast with the other methods, ACOS scores kept progressively increasing at 21 days compared with 14 days (8.21±1.23 versus 2.55±0.69, P<0.01) and at 35 days compared with 14 days (11.61±2.03 versus 2.55±0.69, P<0.001). The average score at 35 days, although representing a 45% increase compared with the 28 days score (7.96±1.97), did not reach statistical difference (P=0.171, Figure 2e). Although, an apparent plateau level was reached at 21 and 28 days, detailed scores for each of the two testing conditions revealed that no plateau of performance was reached with ACOS, at least when treadmill testing was employed (see Figure 3e).
Spontaneous recovery assessed with the ACOS method. Evaluation of spontaneous recovery with a semiquantitative locomotor scale called ACOS. The method is a combination of different assessed parameters: (a) NLM, (b) LM and (c) Amplitude and (d) Incidence. NLMs and LMs are found to significantly increase at 14 days and 21 days, respectively. Amplitude and incidence reached plateaus after 21 days. At this time point, nearly all mice produced NLMs and 50% of them displayed LMs. Compared with scores at day 3, ACOS scores showed significant differences at 14 days versus 35 days (a Friedman test followed by a Dunn's Multiple Comparison post hoc). Differences were found also between 14 days and 21 days and between 28 days and 35 days. #P<0.05, *P<0.01, **P<0.001. Data were pooled from open-field and treadmill conditions
Differences between open-field and treadmill conditions. Two-way repeated measure ANOVA did not reveal task-related differences between open-field and treadmill conditions except for (c) BMS scale where in open-field, score levels are significantly higher (P=0.009) than on treadmill. However, when using a Student paired t-test, differences between conditions were found at some time points for (b) AOB, (d) MFS and (e) ACOS. For all scales, except ACOS, before 14 days, scores tended to be lower in open-field than on the treadmill. From 21 days up to 35 days, scores were higher in open-field conditions. Mice evaluated with ACOS tended to have higher scores on the treadmill throughout the testing period, except at 21 days where, in open-field conditions, scores were higher compared with the treadmill. **P<0.01, *P<0.001
As mentioned in the Methods, the ACOS score is the result of a combination of four distinct factors assessed separately – NLM and LM Frequency, Amplitude and Incidence. In Figure 2a–d, these values were found to progress differently throughout the time period studied. NLM (frequency/min) was found to reach 0.64±0.17 at 7 days, which is almost significantly (P=0.051) different than controls at 3 days (0.09±0.04, Figure 2a). Significantly different values were found at 14 days and later. The highest average value was observed at 35 days with 8.84±1.34 NLMs. NLMs were found to progressively increase with time up to 35 days, except for a small nonsignificant reduction at 28 days. LMs were also observed to increase during the time period studied. While virtually no LM was found at 3 and 7 days post-Tx (except in one mouse where one LM was found), LMs began to be detected at 14 days with an average score of 0.17±0.10 (Figure 2b). Increases were found reaching significantly different values at 21 (0.81±0.26, P<0.01), 28 (1.67±0.81, P<0.05) and 35 days (1.13±0.32, P<0.001) compared with controls (no LM). However, no significantly progressive increase was found given the low average frequency values reported. One reason for this is illustrated in Figure 2d where LMs were found in less than 60% of the mice tested at 21, 28 or 35 days (which considerably affected the average values). In contrast, NLMs were found in nearly all mice tested (95%) at 21 days and later. Figure 2c shows that the assessed amplitudes of all movements (NLMs and LMs) values remained in the lower range of the scale (ie 1-small amplitude and 2-large amplitude, see Methods).
Comparison of methods
Side-by-side comparisons clearly show that scores differed mainly between the ACOS system and the other methods. This is illustrated in Figure 3, where the BBB, AOB, BMS and MFS scales provided virtually identical scores. As mentioned earlier, scores with these four methods significantly increased up to 21 days, after which a plateau was reached (see also Figure 1). Overall, these methods provided values lower than 2 – one of the highest scores was 2.13±0.30 obtained with the AOB scale in open-field conditions at 21 days (see Figure 3b, Table 2). The ACOS method clearly provided significantly higher scores than those obtained with the other methods. Figure 3e illustrates that in open-field conditions, ACOS reached its highest average value at 35 days (8.97±2.78). However, the highest ACOS score was found in treadmill conditions at 35 days with 14.25±2.93 (Figure 3e). Correspondingly, linear regression analyses revealed that significantly steeper slopes were found with the ACOS method compared with the other scales tested (Table 1). In open-field conditions, a near five-fold increase was found with the ACOS method (slope values of 0.28 with ACOS versus <0.07 with any of the other methods, P=0.002, Table 1 and Figure 3a). In treadmill conditions, a ten-fold increase in slope was reported between ACOS and the other methods (0.46 with ACOS versus <0.04 with the others, P<0.001, Table 1 and Figure 3b). Comparison of linear regression slopes clearly showed that ACOS scores increased over a wider range of scoring levels than the other methods during the time period studied. As said, ACOS offers a better discriminative power than the other methods for assessing progressively increasing performances in this animal model, especially in treadmill conditions.
Difference between open-field and treadmill conditions
In general, no difference was found between open-field and treadmill conditions, using the BBB scale. However, a few nonsignificant differences were detected. For instance, at 21 days post-Tx, average BBB scores were greater (P=0.119) in open-field (open circles) than in treadmill (filled circles) conditions (Figure 3a, 1.66±0.25 versus 1.14±0.18). A similar difference (P=0.080) was found at 28 days (1.81±0.20 versus 1.35±0.20, respectively, see also Table 1). With the AOB scale no overall significant difference between conditions was observed. However, significantly different scores (P=0.014) were found at 21 days in open-field versus treadmill conditions (2.13±0.30 versus 1.27±0.17, Figure 3b). Comparable differences with the MFS scale were found at 21 days between open-field and treadmill conditions (Figure 3d). Significant difference between the two tasks was only found with the BMS scale (P=0.009) where at 21 (P=0.013) and 28 days (P=0.001) difference reached significant levels (Figure 3c). Therefore, in general, scores were higher in open-field than in treadmill conditions from 21 to 35 days post-Tx. In turn, average scores were lower in open-field than in treadmill conditions at 3, 7 and 14 days post-Tx although no statistically different levels were reached. In contrast, the ACOS method displayed average scores that were consistently higher in treadmill than in open-field conditions – except at 21 days. However, these differences reached significant (P=0.014) levels only at 14 days post-Tx (Figure 3e). Methods giving highest average scores were ACOS (14.25±2.93: treadmill at 35 days) followed by AOB (2.13±0.30: open-field at 21 days) (Table 2). It is worth noting also that progression of performance assessed with the ACOS method was clearly different in open-field versus treadmill conditions. As mentioned earlier, the linear regression slopes in open-field and treadmill conditions were steeper than those of all the other methods tested (Table 1). However, a close-to-perfect linear relationship (r2=0.99, slope=0.46) reflecting a steady increase of performance over the 5-week period was found on a treadmill, whereas some plateau level was reached in open-field conditions since ACOS scores at 21, 28 and 35 days post-Tx were nonsignificantly different (P>0.05) (Table 2).
Differences between male and female
No systematic difference was found between male and female animals. However, a few punctual differences were found at some time points. For example, at 21 days (P=0.130) higher scores were found in females with the AOB scale (Figure 4b, filled squares), whereas at 14 days higher (P=0.029) scores were found in males with the ACOS method (Figure 4e, open triangles). Otherwise, no significant differences were found between males and females as clearly illustrated by the graphs (Table 3).
Discussion
The results showed that a limited but significant increase of locomotor function recovery can occur without intervention, in completely spinalized mice, tested in open-field or treadmill conditions. Generally, hindlimb movements, assessed with the BBB, AOB, BMS and MFS scales, corresponded to scoring levels lower than 2 (ie no weight-bearing stepping, propulsive or large movements or plantar foot placement) that reached a plateau at 14 or 21 days post-Tx. Only the ACOS scoring system was discriminative enough to detect progressive changes up to 35 days post-Tx, especially in treadmill conditions. None of the methods provided significantly different scores between males and females or between open-field and treadmill conditions, albeit few exceptions.
Possible mechanisms underlying spontaneous recovery without intervention
As summarized above, the results clearly demonstrated the existence of spontaneously occurring hindlimb movement in completely spinal cord Tx mice. These data further confirm the results of previous studies that have reported significant spontaneous recovery in complete Tx rats, cats and mice. Indeed, average scores up to level 5 were assessed after a few weeks with the 22 level-AOB scale in untreated but tail-stimulated spinalized rats examined on a motor-driven treadmill.13 Spontaneous full weight-bearing steps at relatively low treadmill speeds have also been found in untrained and tail-stimulated spinal cats after a few weeks post-Tx.14 In spinalized mice, weight-bearing steps and plantar foot placements have been reported in a few animals. Tests were performed at relatively low-speeds on a motor-driven treadmill, employing tail stimulation and using quantitative kinematic analysis.15 Without intervention (ie no graft, drug treatment, tail stimulation or body-weight support), small but significant levels of spontaneous recovery have been found in all Tx mice tested in open-field conditions.9 Indeed, it was reported that rhythmic and bilaterally alternated movements of small amplitude (ie locomotor-like but with no weight-bearing and plantar foot placement capabilities) spontaneously occur after a few weeks in these animals. The lower level of recovery reported by Guertin9 is in contrast with that reported by Leblond et al.15 This discrepancy was first attributed to different testing conditions (open-field versus motor-driven treadmill, see Discussion in Guertin;9 Guertin and Steuer).18 However, the present results provide additional insights regarding this discrepancy. Indeed, the Tx mice tested on a motor-driven treadmill (at speeds similar to those used by Leblond et al15) were found, with several assessment methods, not to reach recovery levels that included large amplitude movements, weight-bearing stepping and plantar foot placements. Therefore, the relatively high level of recovery reported by Leblond et al15 was most likely attributable to afferent-induced activation of the central pattern generator (CPG), caused by tail stimulation.21 We cannot exclude the possibility that, in both studies, some of the recovery was facilitated or partially caused by repeated testing sessions over time which could constitute some form of training-induced effects upon CPG reorganization and activation.14 However, arguing against this possibility is the fact that we tested the mice only 2 × 4 min per week (ie 4 min in open-field and then in treadmill conditions). This suggests that the spontaneous recovery described, at least in this study, is due to spontaneous sublesional neuronal network changes (eg reorganization and plasticity) and increased CPG excitability. The idea that plasticity at sublesional levels post-Tx may be associated with recovery is supported by results from in vitro preparations showing signs of increased CPG excitability and spontaneous fictive locomotor activity in rodent isolated spinal cords.22, 23 Although it would be beyond the scope of this paper to discuss the cellular mechanisms underlying such spontaneous recovery in Tx animals, recent results suggest a role for specific immediate early genes such as c-fos, Nor-1 and Nur77 as early genetic events that may lead to locomotor network reorganization and spontaneous activation.24 Other transmembranal changes such as increased expression of subsets of serotonin, noradrenaline, glutamate and glycine receptors have also been proposed as factors that may contribute to functional recovery in Tx animals.25, 26, 27
Why would performances ‘level off’ after 2–3 weeks with most assessment methods?
It is clear that small recovery levels such as those seen in this animal model, which are characterized mainly by small amplitude movements, can rarely reach BBB scores above level 1 or 2. This is because large amplitude movements at one or two joints are required to reach levels 2 and 3, respectively.4 A similar limiting factor is found with the BMS scale, given that small amplitude movements generally do not qualify for level 2 (ie extensive ankle movement). If in a few animals, larger movements at the ankle joint were observed, the performances would most certainly reach a plateau at level 3, characterized by either plantar foot placement or dorsal stepping (see BMS scale in Engesser-Cesar et al8). With the MFS scale, level 2 is relatively easy to reach with the type of performance generally observed in the conditions of the present study. Indeed, the second MFS level is only characterized by any movement at one or more joints in one or both limbs. However, a substantial increase of performance is necessary to qualify as level 3 (ie alternate stepping and propulsive movement of hindlimbs),7 making it very difficult to reach that level in the case of spontaneous recovery without intervention. Also, the word propulsive in the MFS scale may be confusing since if no body-weight support is displayed, then it is difficult to associate any spontaneous hindlimb movement with propulsion per se. Regarding the AOB scale, higher scores than with the above methods were generally reported. This may be explained by the fact that this scale was specifically designed for completely spinal cord Tx animals.10 Therefore, level 2, which is defined by weak amplitude movements, can easily be reached by the type of performance found in this study. Level 3 is also easier to reach than with the other methods, given that it requires larger amplitude movements but not necessarily specifically at the ankle joint or at more than one joint or that qualify as stepping (ie no bilateral alternation required). Therefore, it is clear that the type of performance observed in Tx mice without intervention cannot be characterized generally by more than 2 scoring levels with the BBB, BMS, MFS scales and, to some extent, the AOB scale.
However, the present results provide evidence suggesting that another factor also contributed to the development of a plateau in the scoring level. The ACOS method and, in particular, one of its factors, incidence, contributed to the initial progressive increase of the assessed performances. Indeed, analysis of the incidence factor showed that only about 50% of the mice tested displayed some hindlimb movement (NLMs) at 7 days and that few displayed coordinated alternating movements (LMs, Figure 2e). Therefore, even if with BBB, BMS, MFS and AOB scales, level 1 can be reached at 7 days by some animals, the fact that only 50% of them displayed some hindlimb movements or NLMs explains the report of average scores closer to 0.5 than to 1.0 (Figure 1a–d at 7 days). In fact, the apparent progressive increase of performance at 14 days compared with 7 days can be almost entirely explained by an increase of incidence (ie the number of mice in which NLMs were observed). Indeed, incidence values for NLMs reached 80% of mice tested at 14 days which, again, nicely fits with the increase of average scoring levels assessed with most of these methods (ie just below 1.0, Figure 1a–d at 14 days). This idea, that a progressive increase of incidence during the first 2 weeks is a determinant factor in the increase of average scoring levels reported with these methods, is supported by the fact that very similar graphs are found both for incidence (Figure 2e, NLMs) and for average BBB, BMS, MFS and AOB scores (Figure 1a–d). In other words, the initial increase of average scoring levels assessed with the BBB, BMS, MFS and AOB methods are mainly associated with an increase in incidence. This also strongly suggests that the plateau level reached at 14 or 21 days may be explained both by the fact that the incidence had reached near 100% (NLMs) and that, as mentioned earlier, scores above level 2 with these methods are nearly impossible to reach.
How and why is ACOS different?
The ACOS method is different than the other tested methods in many ways. For instance, it is a semiquantitative rather than an entirely qualitative method, given that NLM and LM frequency (counts per min) and incidence are calculated. Not only does this allow the assessment of a factor rarely examined by the other methods (ie frequency), but it also allows performances to be plotted against a wider range of Y-axis values. Consequently, this makes it a more sensitive scale to report the progression of locomotor function recovery (ie movement frequency). Thus, performance regarding NLMs, progressively increased from average scores of ‘0’ (at 3 days) up to ‘9’ (at 35 days, Figure 2a). Another difference of the ACOS method is that bilaterally coordinated movements (LMs) are reported separately to noncoordinated movements (NLMs). Although these two factors are recognized by many of the other methods as distinctive characteristics of functional recovery (eg scores <2 or 3 with the MFS or AOB, respectively, correspond to our definition of NLMs and not LMs),7, 10only the ACOS method allows their evaluation and quantification separately and in parallel. This allowed the finding that NLMs progressed differently than LMs during the time period studied (Figure 2a versus b).
Also, as mentioned earlier, the assessment of incidence both for NLMs and LMs provided information not available with the other tested methods. The quantification of movement amplitude, although performed with arbitrary values (ie 1 – small amplitude, 2 – large amplitude), revealed an important aspect of the performances found in this study. This was that spontaneously occurring movements in Tx mice during this 5-week period do not qualify as large amplitude movements (Figure 2c). All the characteristics or factors assessed with the ACOS method (ie, NLM and LM frequency, amplitude and incidence) when combined to obtain the ACOS score, contributed to produce increasing average scores reflecting generally a progressive increase of the performances during the entire time period studied. This is true if average scores obtained in both testing conditions were averaged as in Figure 2d. Surprisingly however, if examined separately, the ACOS scores in open-field conditions were found to differ considerably compared with those on a treadmill (Figure 3e, Table 1). In open-field conditions, the average scores were found to reach a plateau not dissimilar to those reported with the other tested methods. In fact, it is only with tests on a treadmill that scores and therefore the assessed performances were found to linearly increase for 5 weeks post-Tx. The reasons for this are unclear. However, it is possible that conditions associated specifically with the treadmill tests (ie the hindlimbs dragging behind with the entire front part including the dorsal paws rubbing continuously against the moving treadmill belt) led to some activation of cutaneous and proprioceptive receptors of the hindlimbs. This could have facilitated the progressive increase of performance given that such afferent inputs are well known to modulate, excite and reset CPG activity. This has been shown in decerebrate and paralyzed cats with Ia- and Ib-proprioceptor afferent or cutaneous afferent stimulation during fictive locomotion.28, 29
Concluding remarks
These results contributed to demonstrate that the ACOS scale, especially in treadmill conditions, provides a more sensitive method for assessing the type of recovery and performance occurring spontaneously without intervention in Tx mice. However, it does not assess many of the detailed characteristics inherent to higher recovery levels such as balance, fine placement of the foot and digits, agility, speed, etc. Therefore, to examine higher levels of locomotor recovery, such as after regeneration and reconnection across the lesion induced by grafts or drug treatments, it may be preferable to combine a number of assessment methods for SCI mice and other species. The idea of combining a number of assessment methods for a more complete evaluation of performance is supported by results that have been published by others.30, 31
References
Schwab ME . Repairing the injured spinal cord. Science 2002; 295: 1029–1031.
Steward O et al. Genetic approaches to neurotrauma research: opportunities and potential pitfalls of murine models. Exp Neurol 1999; 157: 19–42.
Kwon BK, Oxland TR, Tetzlaff W . Animal models used in spinal cord regeneration research. Spine 2002; 27: 1504–1510.
Basso DM, Beattie MS, Bresnahan JC . A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12: 1–21.
Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L . Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 2002; 22: 6570–6577.
Basso DM . Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma 2004; 21: 395–404.
Farooque M . Spinal cord compression injury in the mouse: presentation of a model including assessment of motor dysfunction. Acta Neuropathol (Berlin) 2000; 100: 13–22.
Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW . Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma 2005; 22: 157–171.
Guertin PA . Semiquantitative assessment of hindlimb movement recovery without intervention in adult paraplegic mice. Spinal Cord 2005; 43: 162–166.
Antri M, Orsal D, Barthe JY . Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci 2002; 16: 467–476.
Wamil AW, Wamil BD, Hellerqvist CG . CM101-mediated recovery of walking ability in adult mice paralyzed by spinal cord injury. Proc Natl Acad Sci USA 1998; 95: 13188–13193.
Ma M, Basso DM, Walters P, Stokes BT, Jakeman LB . Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp Neurol 2001; 169: 239–254.
Antri M, Barthe JY, Mouffle C, Orsal D . Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett 2005; 384: 162–167.
de Leon RD, Hodgson JA, Roy RR, Edgerton VR . Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol 1998; 79: 1329–1340.
Leblond H, L'Esperance M, Orsal D, Rossignol S . Treadmill locomotion in the intact and spinal mouse. J Neurosci 2003; 23: 11411–11419.
Guertin PA . Role of NMDA receptor activation in serotonin agonist-induced air-stepping in paraplegic mice. Spinal Cord 2004a; 42: 185–190.
Landry ES, Guertin PA . Differential effects of 5-HT(1) and 5-HT(2) receptor agonists on hindlimb movements in paraplegic mice. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 1053–1060.
Guertin PA, Steuer I . Ionotropic 5-HT3 receptor agonist-induced motor responses in the hindlimbs of paraplegic mice. J Neurophysiol 2005; 94: 3397–3405.
Guertin PA . Synergistic activation of the central pattern generator for locomotion by l-beta-3,4-dihydroxyphenylalanine and quipazine in adult paraplegic mice. Neurosci Lett 2004b; 358: 71–74.
Basso DM, Beattie MS, Bresnahan JC . Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996; 139: 244–256.
Strauss I, Lev-Tov A . Neural pathways between sacrocaudal afferents and lumbar pattern generators in neonatal rats. J Neurophysiol 2003; 89: 773–784.
Bonnot A, Morin D, Viala D . Genesis of spontaneous rhythmic motor patterns in the lumbosacral spinal cord of neonate mouse. Brain Res Dev Brain Res 1998; 108: 89–99.
Norreel JC, Pflieger JF, Pearlstein E, Simeoni-Alias J, Clarac F, Vinay L . Reversible disorganization of the locomotor pattern after neonatal spinal cord transection in the rat. J Neurosci 2003; 23: 1924–1932.
Landry ES, Guertin PA . Profile of immediate early gene expression in the lumbar spinal cord of paraplegic mice. Soc Neurosci Abstr 2005; 865.2.
Talmadge RJ, Roy RR, Edgerton VR . Alterations in the glycinergic neurotransmitter system are associated with stepping behavior in neonatal spinal cord transected rats. Soc Neurosci Abstr 1996; 380.
Giroux N, Rossignol S, Reader TA . Autoradiographic study of alpha1- and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol 1999; 406: 402–414.
Ung RV, Landry ES, Lapointe N, Rouillard C, Levesque D, Guertin P . Expression, activation, and function of 5-HT2A receptor in the lumbar spinal cord of adult paraplegic mice. Soc Neurosci Abstr 2005; 516.7.
Guertin P, Angel MJ, Perreault MC, McCrea DA . Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 1995; 487: 197–209.
Perreault MC, Angel MJ, Guertin P, McCrea DA . Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol 1995; 487: 211–220.
Kunkel-Bagden E, Dai HN, Bregman BS . Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp Neurol 1993; 119: 153–164.
Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K . Efficient testing of motor function in spinal cord injured rats. Brain Res 2000; 883: 165–177.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ung, R., Lapointe, N., Tremblay, C. et al. Spontaneous recovery of hindlimb movement in completely spinal cord transected mice: a comparison of assessment methods and conditions. Spinal Cord 45, 367–379 (2007). https://doi.org/10.1038/sj.sc.3101970
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101970
Keywords
This article is cited by
-
Effect of Gender on Recovery After Spinal Cord Injury
Translational Stroke Research (2013)
-
Non-assisted treadmill training does not improve motor recovery and body composition in spinal cord-transected mice
Spinal Cord (2010)