Abstract
Study design:
Single trial using matched subjects under tightly-controlled experimental conditions.
Objective:
Humans with spinal-cord injury have a reduced ability to dissipate heat. The current project examined the possibility that, in such people, an elevated ventilatory response (panting) may act as a supplementary avenue for heat loss.
Setting:
Australia, New South Wales.
Methods:
Breathing frequency was measured during a resting heat exposure (⩽2 h) in 10 subjects with spinal-cord injury (C4–L5), and in 10 mass- and age-matched, able-bodied subjects.
Results:
Subjects with spinal-cord injury displayed a ventilatory sensitivity, relative to mean body temperature change (2.4 breaths/min/°C ±0.9), more than twice that of able-bodied subjects (1.1 breaths/min/°C ±0.6; P=0.042). Furthermore, the higher the level of spinal-cord injury, the greater was the ventilatory response (r2=0.51, P=0.048).
Conclusion:
While these ventilatory changes were apparently thermally mediated, they did not represent a true panting response, nor did the increased breathing frequency confer a physiologically significant thermoregulatory benefit that may help compensate for the loss of sympathetic flow to eccrine sweat glands and cutaneous blood vessels in people with spinal-cord injury.
Similar content being viewed by others
Introduction
Panting is the primary avenue for evaporative heat loss in most nonsweating animals, dissipating as much as 95% of metabolic heat.1 Panting is dominated by an increased breathing frequency, with elevated evaporative heat loss occurring within the upper airways at frequencies as high as 200 breaths/min.2 Humans have an extremely powerful sweating response, and while the coexistence in humans of hyperthermia and tachypnoea has been known for almost 100 years,3 ventilatory heat losses generally constitute a small portion of total heat loss during thermal stress.4 However, people with spinal-cord injury have a reduced ability to regulate body temperature, due to impaired innervation of both sweat glands and cutaneous blood vessels.5, 6, 7 As an alternative, or perhaps supplementary heat loss mechanism, an amplification of the existing, minimal ventilatory (panting) response may occur. Thus, we sought evidence for physiologically significant panting in humans with spinal-cord injury during a resting heat exposure.
While heat-induced tachypnoea, and even hyperventilation,8 is well established in humans, its presence and magnitude in people with spinal-cord injury is less clear.9, 10, 11, 12 Many studies that have addressed this possibility suffer methodological limitations related to controlling the thermal load, quantification of thermal strain or experimental power. Therefore, we revisited this question, but did so using a more powerful design, methods that enabled more precise control over the thermal load, a detailed quantification of thermal strain, and we minimised physiological and medical artefacts. Furthermore, we aimed to quantify the physiological significance of the ventilatory response on heat loss. We hypothesised that subjects with a spinal-cord injury would display a panting response (tachypnoea) in excess of that observed in matched, able-bodied subjects, and that this adaptation might enhance heat dissipation. This possibility was not an unrealistic expectation, since it is known that panting responses can be elicited in animals using locally applied heat to either the hypothalamus or the spinal cord.13
Methods
Subjects and preliminary measures
In total, 10 subjects (eight male, two female) with clinically confirmed, complete spinal-cord injury (C4–L5), and 10 able-bodied males (controls) participated. Both groups were matched for age (36.8 years, SD 8.5) and body mass (77.3 kg, SD 2.6; P>0.05). Subjects with spinal-cord injury received a physical and medical examination before testing, in accordance with the International Medical Society of Paraplegia.14 All subjects provided written, informed consent, and all procedures were approved by the Human Research Ethics Committee (University of Wollongong).
As the time since spinal injury, and the completeness of the injury, may affect physiological adaptation, subjects were recruited from a population in whom the spinal injury had occurred at least 5 years prior to testing (mean 17 years). Clinical verification of injury completeness was initially performed by a specialist physician, confirming the absence of detectable sensory and motor function below the level of injury. Additional verification was achieved by clamping mean body temperature above the threshold for sweating, using a water-perfusion suit and climate chamber.15 The venous return from one leg was then occluded, and the leg cooled (water-filled leg and foot splint; 7.9°C). A decrease in forehead sweat rate during leg cooling was interpreted to indicate that sensory connections across the site of the spinal-cord injury still existed. This was evident in one subject, who was removed from analyses where a quantification of neural deprivation was required.
Subjects were instructed to refrain from alcohol, caffeine, tobacco and strenuous exercise, and to consume a normal diet for the 24 h preceding experimentation. All trials were performed at the same time of the day. Medications known to affect thermoregulation were restricted prior to each trial to minimise residual physiological affects. For example, oxybutynin, a drug with an anticholinergic effect used to prevent muscle spasms, was restricted for 48 h prior to experimentation.
Major causes of autonomic dysreflexia, and the associated nonthermal sweating and ventilatory changes, are bladder and bowel distension, and local tissue ischaemia. Thus, subjects urinated and defecated prior to each trial. To limit pressure-induced ischaemia, all subjects rested on a dry floatation cushion (ROHO© Inc., Belleville, IL, USA). The positioning of tubing and cables between the subject and floatation cushion was minimised, and subjects were moved regularly to reduce localised pressure effects. These precautions minimised both the incidence and severity of spasms throughout the trial. All data relating to pressure relief or spasm, and the 5 min following, were excluded from analysis.
Heat stress tests
Subjects rested seminude (supine), in a climate-controlled chamber at 27.9°C (50.7% relative humidity) for 15 min while baseline data were collected. Mean body temperature was clamped using a water-perfusion suit (water temperature 28.1°C, SD 0.4). This suit consisted of 80 1-m lengths of polyvinyl tubing (Tygon®; I.D.=1.6 mm, O.D.=3.0 mm) arranged in parallel, with adjacent tubes clipped together at 8-cm alternate intervals to form a diamond-shaped lattice. The suit was made up of trousers (30 tubes) and a long-sleeved jacket (50 tubes). After baseline data collection, the climate chamber and suit temperatures were ramped to 38.5°C (38.5% relative humidity) and 38.8°C (SD 0.5), respectively, to provide a stable thermal load for 120 min,15 or until volitional termination (N=2).
Core temperature was measured continuously from the oesophagus (∼40 cm from the nares16), rectum (∼10 cm beyond anal sphincter) and auditory canal (∼20 mm beyond the meatus, and insulated). Skin temperatures were measured from 14 sites, with mean skin temperature calculated using surface area weightings.17 Mean body temperature (Tb) was calculated as follows: Tb=0.8 × oesophageal temperature +0.2 × mean skin temperature. The ventilatory response was assessed from breathing frequency changes, measured using a mercury strain gauge secured to the abdomen and lower ribs (Hokanson EC-4SB, USA). Inspiration was detected from increased electrical resistance of the strain gauge. This technique negated ventilatory modifications commonly associated with breathing masks or mouthpieces.
Analyses
The ventilatory (panting) response was evaluated within subjects from first-order (linear) changes in breathing frequency relative to increments in body temperature. Mean slopes, weighted according to the number of coordinates and the heat-induced range of the mean body temperature change, were obtained for each group. A two-tailed, two-sample z-test was used to evaluate slope differences. Data were then averaged and pooled over 1-min intervals, and a linear regression applied to the mean data for each group. To minimise the effects of differences in starting body temperature, analyses using changes in mean body temperature with breathing frequency were also performed. Within the spinal-cord injured subjects, linear analysis was performed using Pearson Product-Moment correlations (including residual analysis) to evaluate the separate effects of the size of the insensate skin area, and the time since injury, on the ventilatory response. Statistical significance was accepted with an alpha level of 0.05. Experimental data are presented as means with standard errors of the means (±SEM).
Results
The current design ensured subjects were in a thermoneutral environment, resulting in a stable body temperature prior to heating (P=0.867). Under these conditions, there were no differences in breathing frequency (spinal-cord injured: 12.1 breaths/min able-bodied: 12.7 breaths/min, P=0.328) or oesophageal temperature (spinal-cord injured: 36.7°C; able-bodied: 36.9°C; P=0.258) between the groups.
The heated water-perfusion garment, in conjunction with the climate-controlled chamber, provided a substantial thermal stimulus with a simultaneous maintenance of homogeneous skin temperatures, without substantially impeding evaporative heat loss. Mean body temperature was increased by 1.7°C (±0.14) in the spinal-cord injured subjects, and 1.2°C (±0.08) in the able-bodied subjects, while the respective mean, terminal breathing frequencies were 18.1 and 12.8 breaths/min. The breathing frequency responses of the subjects with spinal-cord injury were more closely associated with increments in the core temperature indices (r2=0.64) than with mean skin temperature (r2=0.47).
The sensitivity of this ventilatory response, with respect to mean body temperature increments, was significantly greater in subjects with spinal-cord injury (2.4 breaths/min/°C ±0.91 versus 1.1 breaths/min/°C ±0.62; P=0.042). Separate analysis of the ventilatory and thermal change scores from baseline yielded an almost identical outcome. Thus, a 1°C increase in mean body temperature in the spinal-cord injured group increased breathing frequency by more than twice that observed in the able-bodied subjects. In addition, subjects with a spinal injury resulting in larger regions of insensate skin experienced significantly greater increases in breathing frequency (N=9; r2=0.51, P=0.048), whereas the time since the injury did not influence the panting response (r2=0.02).
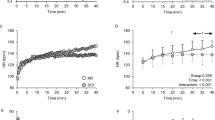
When data were treated within 1-min epochs, and averaged across subjects but within groups, the subjects with spinal-cord injury displayed a significantly greater increase in breathing frequency over both time (Figure 1: r2=0.71; P<0.001) and mean body temperature (Figure 2: r2=0.58; P<0.001). On average, these subjects increased breathing frequency by 50%, from 12 to 18 breaths/min over the heat exposure. This change was bi-phasic in nature, being most evident only beyond a mean body temperature of about 36.9°C (Figure 2), which represented an increase of 0.97°C relative to baseline. However, this apparent threshold was less evident within individual data. The control subjects did not display such a ventilatory response across either time (r2=0.05) or mean body temperature increments (r2=0.03).
Discussion
In this experiment, we manipulated core and skin temperatures, using a precisely controlled exogenous thermal stimulus.15 We used valid and reproducible measures of physiological function, and implemented rigorous screening and standardisation procedures to minimise the possible impact of physiological or pharmacological artefacts on our experimental data. Under these conditions, we have demonstrated a mild, yet significant ventilatory response (tachypnoea) in heated subjects with a long-standing spinal-cord injury, and subsequent thermoregulatory impairment, which was not evident in matched able-bodied subjects. This response was significantly correlated with changes in core temperature, and it appeared bi-phasic in nature across the body temperature range 35.9–37.5°C (Figure 2). Furthermore, subjects with larger areas of insensate skin exhibited a more pronounced panting response.
The magnitude of the thermal strain imposed in this investigation (1.7°C) was beyond the day-to-day mean body temperature range typically encountered by most people with a spinal-cord injury, and similar to temperatures obtained in human panting studies.18, 19, 20, 21 It is therefore reasonable to assume that a substantial, thermally-induced physiological adaptation, if present, should have been revealed within the current project.
While we did observe a significant ventilatory response in subjects with spinal-cord injury, the cause of this response is currently unclear. It may relate to changes in carbon dioxide sensitivity with heating,21 catecholamine production,22, 23, 24 a direct interaction of the preoptic anterior hypothalamus on respiratory control centres,25, 26, 27, 28 or some combination of these factors.
The ventilatory response currently observed could not be described as rapid and shallow breathing. Thus, it was unlikely to be a true panting response. In addition, it did not represent a significant thermoregulatory adaptation. For this to be a possibility, panting should be extensive, and result in physiologically significant heat loss. The panting sensitivity of the dog can exceed 50 breaths/min/°C,29 or 20 times that currently observed. Previous studies have shown ventilatory heat loss, even during exercise, to be almost negligible in able-bodied people.4, 30 Nevertheless, we estimated the additional heat loss that would accompany these ventilatory changes in our spinal-cord injured subjects, using changes in breathing frequency and a standardised tidal volume. These calculations revealed this added heat loss to be equivalent to evaporating sweat at a rate of ∼3 g/h. Given that able-bodied sedentary subjects can readily sustain a sweat secretion of ∼1800 g/h over a 120-min period, we concluded that, while our subjects with spinal-cord injury displayed a statistically significant, and divergent, ventilatory reaction to heating, this response probably lacked thermoregulatory significance. Theoretical calculations show that resting subjects of the same stature, with a metabolic rate of approximately 115 W, would require an additional evaporation of 160 g/h of sweat to prevent a rise in core temperature. The thermal insignificance of the additional ventilatory evaporation is highlighted by the inability of these subjects to regulate the rise in core temperature during this heat exposure.
To minimise ventilatory artefacts on tidal volume, we quantified the ventilatory response wholly from changes in breathing frequency. Since some studies have shown that thermally induced ventilatory responses are associated with increases in tidal volume,8, 31 we cannot exclude the possibility that our subjects also increased tidal volume. However, if we recalculate ventilatory heat loss using our breathing frequency data, and tidal volume changes from the existing literature (900 ml at a mean body temperature of 39.0°C8), we still obtain an almost identical outcome.
In summary, our subjects with spinal-cord injury have displayed a greater breathing frequency response than our able-bodied subjects to an equivalent heat stress. Subjects with a substantially greater reduction in heat loss capacity displayed a more pronounced ventilatory response. However, since this change did not fulfil a meaningful thermoregulatory function, it is concluded that such a mild ventilatory response was unlikely to represent a thermal adaptation in humans with spinal-cord injury.
References
Kamau JM . Metabolism and evaporative heat loss in the dik–dik antelope (Rhynchotragus kirki). Comp Biochem Physiol A 1988; 89: 567–574.
Bligh J . A comparison of the temperature of the blood in the pulmonary artery and in the bicarotid trunk on thermoregulatory mechanisms in the pig. J Physiol 1957; 136: 404–412.
Haldane JS . The influence of high air temperatures. J Hyg 1905; 55: 495–513.
Mitchell JW, Nadel ER, Stolwijk JA . Respiratory weight losses during exercise. J Appl Physiol 1972; 32: 474–476.
Wallin BG, Stjernberg L . Sympathetic activity in man after spinal cord injury. Outflow to skin below the lesion. Brain 1984; 107: 183–198.
Sawka MN, Latzka WA, Pandolf KB . Temperature regulation during upper body exercise: able-bodied and spinal cord injured. Med Sci Sports Exerc 1989; 21: S132–S140.
Muraki S, Yamasaki M, Ishii K, Kikuchi K, Seki K . Relationship between core temperature and skin blood flux in lower limbs during prolonged arm exercise in persons with spinal cord injury. Eur J Appl Physiol 1996; 72: 330–334.
Gaudio R, Abramson N . Heat-induced hyperventilation. J Appl Physiol 1968; 25: 742–746.
Guttmann L, Silver J, Wyndham CH . Thermoregulation in spinal man. J Physiol 1958; 142: 406–419.
Johnson RH . Temperature regulation in paraplegia. Paraplegia 1971; 9: 137–145.
Totel GL, Johnson RE, Fay FA, Goldstein JA, Schick J . Experimental hyperthermia in traumatic quadriplegia. Int J Biometeorol 1971; 15: 346–355.
Totel GL . Physiological responses to heat of resting man with impaired sweating capacity. J Appl Physiol 1974; 37: 346–352.
Guieu JD, Hardy JD . Effects of preoptic and spinal cord temperature in control of thermal polypnea. J Appl Physiol 1970; 28: 540–542.
Maynard FM, Bracken MB, Creasey G, Ditunno JF, Donovan WH, Ducker TB . International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
Cotter JD, Patterson MJ, Taylor NAS . A method for clamping human skin and body core temperatures. Proc Aust Physiol Pharmacol Soc 1995; 26: 204p.
Mekjavic IB, Rempel ME . Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol 1990; 69: 376–379.
ISO. Evaluation of Thermal Strain by Physiological Measurements. International Standards Organisation, Geneva. Report number 9886, 1992.
Vejby-Christensen H, Strange Petersen E . Effect of body temperature and hypoxia on the ventilatory CO2 response in man. Resp Physiol 1973; 19: 322–332.
Saxton C . Respiration during heat stress. Aviat Space Environ Med 1975; 46: 41–46.
Petersen ES, Vejby-Christensen H . Effects of body temperature on ventilatory response to hypoxia and breathing pattern in man. J Appl Physiol 1977; 42: 492–500.
Baker JF, Goode RC, Duffin J . The effect of a rise in body temperature on the central-chemoreflex ventilatory response to carbon dioxide. Eur J Appl Physiol 1996; 72: 537–541.
Hussi E, Sonck T, Poso H, Remes J, Eisalo A, Janne J . Plasma catecholamines in Finnish sauna. Ann Clin Res 1977; 9: 301–304.
Laatikainen T, Salminen K, Kohvakka A, Pettersson J . Response of plasma endorphins, prolactin and catecholamines in women to intense heat in a sauna. Eur J Appl Physiol 1988; 57: 98–102.
Brenner I, Shek PN, Zamecnik J, Shephard RJ . Stress hormones and the immunological responses to heat and exercise. Int J Sports Med 1998; 19: 130–143.
Hammel HT, Hardy JD, Fusco MM . Thermoregulatory responses to hypothalamic cooling in unanaesthetised dogs. Am J Physiol 1960; 198: 481–486.
Baldwin BA, Ingram DL . The influence of hypothalamic temperature and ambient temperature on thermoregulatory mechanisms in the pig. J Physiol 1968; 198: 517–529.
Hales JRS, Bennett JW, Fawcett AA . Integrated changes in regional circulatory activity evoked by thermal stimulation of the hypothalamus. Pflugers Arch 1977; 372: 157–164.
Ni H, Zhang J, Glotzbach SF, Schechtman VL, Harper RM . Dynamic respiratory responses to preoptic/anterior hypothalamic warming in the sleeping cat. Sleep 1994; 17: 657–664.
Hales JR, Kao FF, Mei SS, Wang C, Gretenstein M . Panting in heated cross-circulated dogs. Am J Physiol 1970; 218: 1389–1393.
Hanson RDG . Respiratory heat loss at increased core temperature. J Appl Physiol 1974; 37: 103–107.
Cabanac M, White MD . Core temperature thresholds for hyperpnea during passive hyperthermia in humans. Eur J Appl Physiol 1995; 71: 71–76.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilsmore, B., Cotter, J., Bashford, G. et al. Ventilatory changes in heat-stressed humans with spinal-cord injury. Spinal Cord 44, 160–164 (2006). https://doi.org/10.1038/sj.sc.3101823
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101823
Keywords
This article is cited by
-
Short-term exercise-heat acclimation enhances skin vasodilation but not hyperthermic hyperpnea in humans exercising in a hot environment
European Journal of Applied Physiology (2012)
-
The cross-sectional relationships among hyperthermia-induced hyperventilation, peak oxygen consumption, and the cutaneous vasodilatory response during exercise
European Journal of Applied Physiology (2009)
-
Thermoregulation in tetraplegic patients
Spinal Cord (2007)