Abstract
Objectives:
The objective of this study was to evaluate the accuracy, reliability, safety, and efficacy of the Codman Model 3000 Constant Flow Implantable Infusion Pump for intrathecal baclofen delivery as a therapeutic option for the treatment of severe spasticity. The distinctive features of this pump include a raised, easily palpable septum, a safety valve protecting the bolus pathway, no programmer needed, and no battery to fail.
Design:
A total of 17 patients with spinal cord injury, multiple sclerosis, or cerebral palsy were implanted with this pump. The accuracy of the pump and drug treatment efficacy was determined at each visit and adjustments to the dosages were made as required. All the intrathecal drug delivery system complications were reviewed.
Results:
The expected efficacy was achieved. The accuracy of the implanted pumps ranged from 90–97% (average 94%). There were no complications due to primary pump problems. The complications reported are similar to other implantable infusion devices and include dehiscence of the suture line, pressure ulcer development, formation of seroma, inversion of the pump, baclofen overdose, and catheter failures.
Conclusion:
The Codman Model 3000 Constant Flow Implantable Infusion Pump is an accurate, reliable, and convenient option for patients needing intrathecal baclofen therapy, with complications similar to other available pumps.
Similar content being viewed by others
Introduction
Spasticity is an abnormal increase of velocity-dependent muscle tone, due to an upper motor neuron lesion. Severe spasticity can, among other things, limit function, interfere with sleep, and cause pain. In those with severe spasticity uncontrolled with oral medication, using baclofen by intrathecal infusion can be very efficacious.1
Direct intrathecal infusion of medications to treat spasticity circumvents some of the common problems associated with oral drugs such as the blood–brain barrier and more widespread systemic and central nervous system toxic effects. Since the introduction of bacteriostatic filters, implantable pumps have been used to provide continuous intraspinal infusion of medications such as baclofen safely.2, 3
The implantable pumps in clinical use utilize two basic propellant technologies: electrical/programmable pumps and mechanical constant flow style pumps. The advantage of the electrical/programmable pump is flexibility of dosage control via the programmer. The advantage of the mechanical style of pump is that it does not need an energy cell and, therefore, does not require replacement due to energy failure. As it is nonprogrammable, it does not require a specialized programmer for refills. A comparison of these styles of pumps has been reported previously.4
There are several mechanical style pumps used previously and presently (eg previously Pfizer's Infusaid, and presently Codman's Model 3000 (Raynham, Massachusetts) and Medtronic's Isomed (Minneapolis, Minnesota)), but only a few reports have appeared in the literature on the clinical use of these pumps for spasticity. There are reports of successful clinical experiences with case series of spasticity treated with the Infusaid mechanical pump, which is no longer available.5, 6, 7 The Codman Model 3000 Constant Flow Implantable Infusion Pump8 (previously known as Arrow 3000 and, before that, Therex 3000) is the focus of this paper. The pumps have changed names over the years, but have remained the same pump, thus whether it was called Arrow 3000 or Therex 3000 at the time, all the pumps are referred to as ‘Codman 3000’ in this document. Previous reports in the literature on this pump are reports of safety of use and efficacy of treatment for regional chemotherapy and pain control.9, 10, 11, 12 This device is disc-shaped, made of titanium and has an internal drug reservoir reached by percutaneous puncture of a silicone rubber septum. The drug chamber is contained within a metal bellows. This chamber has a compressible fluorocarbon propellant in a second outer chamber. Filling the drug reservoir compresses the propellant, creating pressure within that chamber. The pressure drives the drug from the collapsible chamber through a capillary restrictor tube, through an antibacterial and particulate filter, and then into the patient's spinal catheter. Flow rates are factory preset and range from about 0.5 to 2.0 ml/day.
The primary purpose of this study was to evaluate the accuracy, reliability, and safety of the Codman Model 3000 Constant Flow Implantable Infusion Pump as a delivery system providing continuous infusion of intrathecal baclofen. Efficacy was measured as a secondary measure rather than a primary measure as multiple previous studies have shown that intrathecal baclofen is efficacious.1, 3, 5, 6, 13, 14, 15, 16, 17, 18, 19, 20, 21
Methods
Subjects
This was a prospective study of people referred for treatment of severe spasticity of the lower limbs due to spinal cord injury (SCI), multiple sclerosis (MS), or cerebral palsy (CP). Subjects were recruited in a consecutive manner from referrals to our clinic.
Ethics approval
Approval for this study was granted by the local research ethics board in October 1994 and recruitment began in November 1994.
Inclusion criteria
Spasticity had been present for at least 12 months. The lower limbs had severe spasticity, although upper limbs could be involved as well. The subjects had not responded to other medications and therapies or side effects had limited their use. Male and female subjects were considered.
Implantation and follow-up procedures
Screening was performed as per usual protocols14, 17, 18 to assess patients for intrathecal baclofen. Once the subject had a successful trial, the Codman 3000 pump was implanted. After implantation, the dosage of the intrathecal drug was increased as the oral medications for spasticity were tapered gradually and discontinued as tolerated by the patient. Decisions were made to increase or decrease intrathecal baclofen dose at each refill according to the subject's symptoms of recent problematic lower extremity spasticity and physical examination including Ashworth scale.
Outcome measures
Primary outcome measures were: (1) accuracy of the pump (calculated at each refill procedure) (see Appendix A), (2) reliability (assessed by ability of pump to maintain pumping for a continuous time), and (3) safety (assessed by tracking any complications). Secondary outcome measure was efficacy of the drug, as measured by subject's ability to wean off oral medication. As the efficacy of intrathecal baclofen has been reported in many papers, serial Ashworth measurements will not be presented here.
Results
Demographics
Between 1994 and 2001, 18 Codman infusion pumps were implanted into 16 patients with severe spasticity. The patients included six female subjects, all of whom had MS, and 10 male subjects, four with MS, five with SCI, and one with CP. Age at the time of implant ranged from 11 to 59 years, average 41 years. The length of time from diagnosis to implant ranged from 18 months to 32 years, average 16 years. The number of months of follow-up since implant ranged from 5 to 76 months, average 41 months. Five patients had programmable pumps previous to the Codman pump implants. Two patients required replacement of their Codman pumps due to infection that occurred when the pumps extravasated.
Primary outcome measures
Accuracy and reliability
The factory-set flow rates of these 18 pumps vary from 0.49 to 1.0 ml/day. The average accuracy of these flow rates as measured at each refill ranged from 90 to 97%, average 94%. The number of refill procedures from implant date to present ranged from 4 to 62 per pump, for a total of 486 refill procedures. As time from implant increased, the accuracy did not change significantly in any of the pumps, that is, the reliability was excellent. None of the pumps had any failures in pumping mechanism or ceased to work at any time.
Safety
There were no failures of the pump itself. This includes no failures of the pump mechanism or of the casing/septum or the interior tubing. Postoperative dehiscence of the suture line at the pump site occurred in two subjects. One occurred when the staples were removed too soon postsurgery. The suture line was reclosed and healed without further incident. In the other subject, the pump extravasated 12 days postimplant of the Codman pump that was replacing an electric style pump in which the battery had expired. One person developed a pressure ulcer over the pump site as a result of friction from an orthotic vest and the pump extravasated. A seroma formed in the pump pocket of one subject a few days postoperatively. One pump inverted within the pocket of an obese person that was corrected by manipulation. The most serious complication was a baclofen overdose that occurred when a subject was given too high a bolus intraoperatively. This person was kept under observation, given supportive treatment and physostigmine, and recovered completely. The most frequent complication was catheter failures. There were three catheter fractures and one catheter kink, all of which required replacement. One catheter appeared to have migrated. This was not replaced, but higher doses of drug were required for optimal effect. Another catheter was replaced after the tip migrated caudally and in two subjects, the catheters became disconnected at a connector site and required surgical reconnection.
Secondary outcome measures
Efficacy
The maximum baclofen dose ranged from 89 to 918 μg per day and averaged 322 μg per day to achieve the desired reduction in muscle tone. Oral baclofen and other antispasticity medication were discontinued in all patients, except those with multiple sclerosis who had bothersome upper limb spasticity.
Discussion
The complications that were experienced are similar to other implantable infusion devices,13, 18, 19, 22, 23, 24, 25 with the exception that there were no failures of the Codman pump mechanism, unlike reports of failures in the programmable pumps.19, 24 Five of the patients had programmable pumps previously and chose to have them replaced with an Codman Constant Flow pump when the programmable pump batteries expired. A programmer is not required, therefore, these refill procedures can be carried out by a family member or other caregiver who can be trained in the technique. A nurse at a long-term care facility and the spouses of two of the patients have been trained and are performing the refill procedures. They then complete the refill form and fax it to the coordinator of the intrathecal pump program for monitoring. For those with a large geographical distance from our centre, this is quite convenient as it allows for intrathecal baclofen therapy in those that would not otherwise be considered for this type of therapy with a programmable pump. It also eliminates frequent travel to our clinic for those living close to our centre that are difficult to transport, such as those in long-term care facilities.
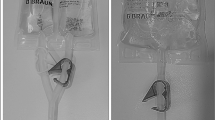
The Codman Constant Flow Infusion pump is designed with a raised septum at the centre of the device that can easily be palpated through the skin, making the refill septum more accessible. This feature also allows for much easier detection should a pump invert within the pocket. It also has a unique built-in safety device. There is only one septum for both the refill procedure and the bolus pathway, but a special bolus needle is required to access the bolus pathway. The opening on the needle used in the refill procedure is located at the tip of the needle (Figure 1). The special bolus needle is closed at the tip and its opening is located part way down the shaft of the needle where the fluid for injection enters the bolus pathway (Figure 2). The special bolus needle and package are well marked to alert the user so that mistakes can be avoided.
The Codman pump's flow rate is factory set and remains constant. The dosage of the drug to be infused is controlled by the concentration of the drug instilled into the pump reservoir. To change the dosage, the pump reservoir is emptied and refilled with an altered concentration. The effect of the altered dosage is not evident until the amount of drug in the catheter between the pump and the intrathecal site has infused and the drug in the altered concentration starts infusing.
The pumping action in an Codman infusion device is based on a two-phase charging fluid that compresses expansion bellows. Therefore, apparently the flow rate increases or decreases by 10–13% per degree Celsius change in body temperature.26 Patients are thus cautioned to avoid saunas and prolonged hot baths and should be assessed with regard to increased flow rates during fevers. Travel in commercial airplanes or prolonged exposure to high altitudes (ie >6000 feet above sea level) may also result in an increase in flow rate by up to 35%.26 Several of the patients in this review have had fevers and have travelled on commercial airlines without any noticeable effects of increase in baclofen dosage. One person flew for 18 h within a 24-h period, also without any problems.
Conclusion
The Codman Model 3000 Constant Flow Implantable Infusion Pump is an accurate, reliable, convenient, and efficacious option for patients needing intrathecal drug therapy. The complication rates are similar to other pumps, and are not pump specific but rather catheter, medication, or wound related.
References
Nance PW, Schryvers OI, Schmidt BJ, Dubo HI, Fewer D . Intrathecal baclofen therapy for adults with spinal spasticity: therapeutic efficacy and effect on hospital admissions. Can J Neurol Sci 1995; 22: 22–29.
Coombs DW, Pageau MG, Saunders RL, Mroz WT . Intraspinal narcotic tolerance: preliminary experience with continuous bupivicane HCL infusion via implanted infusion device. Int J Artif Organs 1982; 5: 379–382.
Penn RD, Kroin JS . Long-term intrathecal baclofen infusion for treatment of spasticity. J Neurosurg 1987; 66: 181–185.
Nance P, Meythaler J . Intrathecal drug therapy. Phys Med Rehab Clin N Am 1999; 10: 385–401.
Zierski J, Muller H, Dralle D, Wurdinger T . Implanted pump systems for treatment of spasticity. Acta Neurochir Suppl (Wien) 1988; 43: 94–99.
Becker WJ, Harris CJ, Long ML, Ablett DP, Klein GM, DeForge DA . Long term intrathecal baclofen therapy in patients with intractable spasticity. Can J Neurol Sci 1995; 22: 208–217.
Gardner B et al. Intrathecal baclofen – a multicentre clinical comparison of the Medtronics Programmable, Cordis Secor and Constant Infusion Infusaid drug delivery systems. Paraplegia 1995; 33: 551–554.
Codman & Shurtleff, Inc. Products: implantable pumps: pump overview. http://www.codmanpain.com/Products_pumps_overview.asp. 2004.
Szakacs JG, Szakacs JE, Karl RC . Surgical resection versus perfusion in the treatment of metastatic and primary tumors. Ann Clin Lab Sci 1990; 20: 245–247.
Coombs DW, Fine N . Spinal anesthesia using subcutaneously implanted pumps for intrathecal drug infusion. Anesth Analg 1991; 73: 226–231.
Kemeny MM . The surgical aspects of the totally implantable hepatic artery infusion pump. Arch Surg 2001; 136: 348–352.
Winkelmuller M, Winkelmuller W . Long-term effects of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. J Neurosurg 1996; 85: 458–467.
Coffey RJ et al. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg 1993; 78: 226–232.
Meythaler JM, Guin-Renfroe S, Law C, Grabb P, Hadley MN . Continuously infused intrathecal baclofen over 12 months for spastic hypertonia in adolescents and adults with cerebral palsy. Arch Phys Med Rehabil 2001; 82: 155–161.
Azouvi P, Mane M, Thiebaut JB, Denys P, Remy-Neris O, Bussel B . Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil 1996; 77: 35–39.
Campbell WM et al. Long-term safety and efficacy of continuous intrathecal baclofen. Dev Med Child Neurol 2002; 44: 660–665.
Meythaler JM, Guin-Renfroe S, Brunner RC, Hadley MN . Intrathecal baclofen for spastic hypertonia from stroke. Stroke 2001; 32: 2099–2109.
Meythaler JM, Steers WD, Tuel SM, Cross LL, Haworth CS . Continuous intrathecal baclofen in spinal cord spasticity. A prospective study. Am J Phys Med Rehabil 1992; 71: 321–327.
Penn RD . Intrathecal baclofen for spasticity of spinal origin: seven years of experience. J Neurosurg 1992; 77: 236–240.
Stempien L, Tsai T . Intrathecal baclofen pump use for spasticity: a clinical survey. Am J Phys Med Rehabil 2000; 79: 536–541.
Dario A, Scamoni C, Bono G, Ghezzi A, Zaffaroni M . Functional improvement in patients with severe spinal spasticity treated with chronic intrathecal baclofen infusion. Funct Neurol 2001; 16: 311–315.
Saltuari L et al. Indication, efficiency and complications of intrathecal pump supported baclofen treatment in spinal spasticity. Acta Neurol (Napoli) 1992; 14: 187–194.
Levin AB, Sperling KB . Complications associated with infusion pumps implanted for spasticity. Stereotact Funct Neurosurg 1995; 65: 147–151.
Armstrong RW, Steinbok P, Cochrane DD, Kube SD, Fife SE, Farrell K . Intrathecally administered baclofen for treatment of children with spasticity of cerebral origin. J Neurosurg 1997; 87: 409–414.
Pasquier Y, Cahana A, Schnider A . Subdural catheter migration may lead to baclofen pump dysfunction. Spinal Cord 2003; 41: 700–702.
Codman & Shurtleff Inc. Codman 3000 Series Constant Flow Implantable Pump with Bolus Safety Valve: Instructions for use, TLB2862M Raynham, MA, USA, pp 25–26.
Author information
Authors and Affiliations
Additional information
This study was conducted at Winnipeg, Manitoba, Canada, and the support for equipment was received from Arrow International, Inc.
Presented at the 3rd World Congress in Neurological Rehabilitation in Venice. Italy, April 2–6, 2002
Appendix A: Pump Refill Record
Appendix A: Pump Refill Record

Rights and permissions
About this article
Cite this article
Ethans, K., Schryvers, O., Nance, P. et al. Intrathecal drug therapy using the Codman Model 3000 Constant Flow Implantable Infusion Pumps: experience with 17 cases. Spinal Cord 43, 214–218 (2005). https://doi.org/10.1038/sj.sc.3101684
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101684
Keywords
This article is cited by
-
Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases
Biomedical Microdevices (2019)