Abstract
Study Design: Comparison of spinal lesion subjects and normal subjects.
Objective: To investigate the effects of a paravertebral repetitive magnetic stimulation on spastic tone increase of the lower limbs.
Setting: Munich, Germany.
Methods: We compared the effects in 15 patients with different spinal lesions and in 16 healthy subjects. The spastic tone increase was evaluated clinically with the Ashworth scale and apparatively with the pendulum test, both at fixed times before and after stimulation. Unilateral stimulation was applied to the lumbar nerve roots L3 and L4 of the clinically more spastic leg.
Results: The spastic tone decreased significantly in the interval between 4 and 24 h after stimulation. This effect was slightly more pronounced in the contralateral extremity. Furthermore, the stimulation motor threshold of the patients was significantly raised.
Conclusion: Repetitive magnetic unilateral stimulation has a positive effect on spastic tone increase due to spinal lesions, causing a decrease that lasts for about 1 day not only on the ipsilateral but also on the contralateral side.
Similar content being viewed by others
Introduction
A lesion of the central descending motor pathways in humans leads to a velocity-dependent increase in muscle resistance during passive stretching. This part of the spasticity complex is called spastic tone increase. It might be caused by an overactivity of the stretch reflexes1 and is seen only in primates. Normally, it develops after a delay of weeks or months. The spastic tone increase often poses a therapeutic problem during rehabilitation, causing joint contractures, and co-contractions with a limited range of motion. Other problems are muscle weakness and painful muscle activity during stretches, sometimes induced by only slight touches of the skin.2 The basic treatment remains physical therapy and pharmacotherapy,3 often in combination.4
Evidence indicates that the sensory feedback produced by passive movements of the affected limb, as well as the activation of commissural pathways due to the activation of interneurons, that is, by moving the contralateral extremity, causes a reduction in spastic tone.3 This also explains why physiotherapy helps.5 Electrical stimulation can also reduce spastic tone,6,7,8 but has not yet been introduced in the clinic as standard therapy due to its various side effects. Recently, two groups showed that repetitive magnetic stimulation has positive effects on spastic tone increase. The repetitive stimulation was applied at peripheral nerves9 to treat spastic tone of the upper limbs. A second group applied serial stimulation at the mid-thoracic level on the spinal cord to treat spastic tone of the lower limbs.10,11 However, the underlying mechanisms are still controversial.
Unlike these previous studies we investigated the possible effects of repetitive magnetic stimulation on spastic tone increase of the lower limbs by applying the stimulation paravertebrally at the lumbar level, thus stimulating spinal nerve roots innervating the targeted muscles, the quadriceps muscle of the thigh.
Methods
Subjects
A total of 16 healthy subjects without any known neurological disorder (mean age 34.2 years, range: 24–59 years) and 15 patients (mean age 42.3 years, range: 23–68 years) with spastic tone increase of the lower limbs due to different spinal diseases participated in the study (Table 1). The underlying diseases included multiple sclerosis with spinal lesions, familial spastic spinal paralysis, transverse myelitis, and spinal vasculitis. The mean duration of spastic tone at the beginning of the study was 4.4 years (range: 1/2–12 years). As the clinical status of the spastic tone remained constant, no changes in therapeutic approach had been made during the last 6 months before stimulation, except for one patient who had spastic tone increase for 1/2 year. His treatment and clinical state was unchanged for about 3 months before testing. All patients and subjects gave their informed consent and the study was approved by the local ethics committee.
Measurements
The spastic tone increase was assessed in two ways: (1) clinically with the modified Ashworth scale12 and (2) technically with a modified form13 of Wartenberg's14 pendulum test of spasticity. The Ashworth scale rates the spastic tone by measuring passive movements of the lower leg against the upper leg. The scale ranges from 0 = no spastic tone, to 4 = most severe tone, in which no movements are possible.
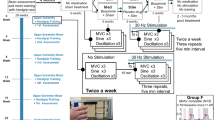
The pendulum test measures the free swing movements of the lower leg against the upper leg with an electrical goniometer, which was placed at the knee. These movements normally consist of a damped pendular swinging. Optimal relaxation is an important prerequisite for valid results. A normal pendulum test is shown in Figure 1 (upper right graph). One patient's pendulum test is shown in the lower right graph. The analogous output of the goniometer data was digitized, stored for off-line analysis on a computer, and analyzed with a customized program using Matlab®. This program allowed a semiautomatic analysis of the knee angles over time.13 Eight parameters in the pendulum test can be calculated from the recorded curve (see Figure 1, graphs). For further evaluation, we used the parameter measuring the peak velocity of the first swing, from a stretched position to the maximum flexion position. This depends on muscle resistance during the passive stretching phase and is measured in deg/s. A total of 10 completed pendulum tests were derived and the mean was calculated.
Setup of the stimulation and pendulum test recording. A typical example of the goniometer recording during a pendulum test in a healthy subject (right upper graph) and a patient with severe spasticity (Ashworth scale of about 3, right lower graph) are shown. The curves describe the range of motion over time
To monitor the extent of muscle relaxation during the pendulum test, we used a surface electromyograph (EMG) of the quadriceps muscle with an acoustic control of voluntary muscle contraction. The surface EMG was always recorded during swinging of the leg.
EMG was also used to determine the stimulation motor threshold and the optimal stimulation location at the back. Both quadriceps muscles were simultaneously recorded to ensure that mainly one side was stimulated. The motor threshold is defined as the stimulation intensity at which five single stimuli applied at the spinal level lead to a consecutive response (larger than 50 μV) on the surface EMG of the quadriceps muscle of the more affected leg. Thus, no motor response was recorded on the other side. However, contralateral subthreshold effects could not be excluded. For this reason, we do not use the terms ‘stimulated and not-stimulated side’, but ‘ipsilateral and contralateral’ stimulation side. The single stimuli as well as the repetitive stimulation did not cause any visible muscle contraction or movements.
Magnetic stimulation
The magnetic stimulation was done with Magstim® Rapid (MAGSTIM® Company, Great Britain) with a maximum output of 1.275 T. We used a circular coil with a diameter of 90 mm positioned at the level of vertebrae L3 and L4 about 2 cm paravertebrally and oriented with the grip vertical to the spinal cord; this is a good coil position for stimulating spinal nerve roots.15 As the stimulator switched the coil off when it became too warm, two magnetic coils were used alternately to apply the full amount of magnetic stimuli in all patients. While determining the intensity for the stimulation motor threshold, we also located the best position and marked it on the subject's back. The intensity of the motor threshold was increased by around 20% for the stimulation series.
Procedure
The subjects were seated at the recording desk (see Figure 1), after having been evaluated using the Ashworth scale and the pendulum test before stimulation. The repetitive magnetic stimulation was applied 10 times, each series of stimulations lasting 10 s at a frequency of 20 Hz. The interstimulus interval was 40 s. Altogether, 2000 single magnetic stimuli were given. The side stimulated was always the clinically more affected leg. Patients were tested directly afterward and then 2, 4, 24, and 48 h later using the Ashworth scale and the pendulum test. The healthy subjects were evaluated only three times, while the preliminary tests showed no changes. All were systematically stimulated on the right side. Pre-experiments included a sham stimulation. It proved problematical to reduce the stimulation intensity to subthreshold levels, because most of the patients perceived the changed stimulation parameter as ‘no stimulation’. Thus, the patients were confident of having had no effect from the sham stimulation. As subjective effects of stimulation, however, could not be completely eliminated, the sham stimulation was not continued in the remaining experiments.
Statistics
The variables submitted to statistical analysis were peak velocity of the first leg swing, stimulation intensity to determine motor threshold, and the Ashworth score. Statistical differences between the pre and post measurements and between patients and controls were tested by Wilcoxon's tests for paired samples and with the Bonferroni correction for multiple comparisons. This analysis was performed separately for ipsilateral and contralateral stimulation sides. Bonferroni correction was carried out separately for each variable because of their theoretical independence. The corrected level of significance for peak velocity and Ashworth score was set at P=0.008. For stimulation intensity, P was set at 0.05.
Results
Healthy subjects
As expected, the modified Ashworth scale was normal; however, the healthy subjects also showed a slight increase in velocity of the first swing in the pendulum test (ipsilateral: 471 °s−1 before to 500°s−1 afterward; P=0.01; contralateral: 439°s−1 before to 475°s−1 afterward; P=0.01). This was recorded directly and 2 h after stimulation (Figure 2, lower right graph). Changes in velocity were not significant and smaller than in the patient group.
Subject data. The mean and standard deviation are shown in black columns for the ipsilateral side and gray for the contralateral side. Statistically significant results are connected with bars and marked with an asterisk (*). (Top) Patient data (n=15 in the first controls; n=11 48 h later) according to the modified Ashworth scale. (Bottom left) Patient data (n=15 in the first controls; n=11 48 h later) for the peak velocity of the first swing of the pendulum test. Note that after 48 h, only 11 patients could be re-tested. (Bottom right) Data of the healthy subjects (n=16) for the peak velocity. The modified Ashworth scale and pendulum test showed a similar pattern that peaked 24 h after stimulation
Patients
In total, 10 patients were stimulated on the right and five on the left side. The modified Ashworth scale showed a significant reduction in spastic tone (see Figure 2, upper graph). This reduction reached a peak in subsequent tests about 4–24 h after stimulation (ipsilateral and contralateral: P<0.008). Clinically there was no decrease in spastic tone directly after stimulation. Only 11 patients could be re-evaluated after 48 h. These showed that the values returned to the prestimulation level for the ipsilateral side. The contralateral side was also decreased.
Pendulum testing also reflected this change in spastic tone increase. The velocity of the first swing of the lower leg increased significantly in both legs after stimulation (ipsilateral: 362°s−1 before to 428°s−1 after 24 h; P<0.008; contralateral: 344°s−1 before to 424°s−1 after 24 h; P<0.008), shown in Figure 2 (lower left graph). The peak velocity showed an increase immediately after stimulation in some patients. However, this increase was not significant (P=0.04 ipsilateral versus P=0.02 contralateral), while the further course showed a second peak after 24 h followed by a decrease after 48 h. Surprisingly, the increase in peak velocity tended to have a wider range on the contralateral side (contralateral 80°s−1 versus ipsilateral 66°s−1; P=0.09). Besides the peak velocity of the second swing and the number of swings made during one test, additional parameters of the pendulum test showed similar behavior for decreased spastic tone.
The intensity for determining the motor threshold was significantly higher in the patient group than in the controls (43% of the maximal stimulator output compared with 32% (P=0.01)). The patients were stimulated with a mean intensity of 0.55 T, and the control subjects with a mean intensity of 0.4 T.
Discussion
This study yielded three main results. First, we also found a significant decrease in spastic tone, seen as an increase in swing velocity of the lower limbs. After unilateral stimulation, this was not only seen on the ipsilateral but also on the contralateral side. Surprisingly, the reduction of spastic tone tended to be more pronounced contralaterally. Second, the decrease started after a few hours and lasted for around 20 h (in controls after 4 and 24 h). Third, the motor threshold for the paraspinal magnetic stimulation was significantly higher in patients than in controls.
Similar effects of repetitive magnetic stimulation in the literature
There are, to our knowledge, only three earlier publications dealing with the effects of repetitive spinal10,11 and peripheral9 magnetic stimulation on spastic tone increase. One study investigated patients with multiple sclerosis after stimulation of the mid-thoracic spinal cord, while the coil was located centrally over the spinal cord. The authors showed that the maximum force in dorsal and plantar flexions of the feet was increased, while the modified Ashworth scale was significantly decreased after stimulation. Since they also investigated the spinal motor excitability (by means of the H-reflex), their hypothesis was that the repetitive magnetic stimulation leads to a long-term depression of synaptic transmission mainly on spinal level.11
The second study investigated the effects of peripheral repetitive magnetic stimulation on spastic tone caused by cerebral infarctions of the upper limbs. Spastic tone was measured by recording the range of motion and the maximum EMG activity of the arms. The results of this study showed that spastic tone decreased immediately after stimulation and was followed by a reduction in spastic tone lasting up to 24 h. The authors hypothesized that they had produced a proprioceptive input to the spinal motor system and to supraspinal centers, which reduced the spastic tone. They further hypothesized that the repetitive magnetic stimulation could inhibit the signal flow and thus modulate spastic tone on the spinal level. They also suggested that cortical mechanisms are involved due to corticocortical connections. Moreover, similar effects were found after stimulating the opposite side. The recorded effects were thought to show a summation of spinal and cortical mechanisms. We also found a reduction of spastic tone after repetitive magnetic stimulation. However, we also detected other effects, which we dicuss below.
Bilateral short- and long-term effects after unilateral root stimulation
We were able to show crossing effects after a unilateral repetitive stimulation, that is, reduced spastic tone was observed not only ipsilaterally but also contralaterally. A similar effect is known from repetitive exercises of the less affected limb. This can lead to a decrease in spasticity even on the opposite side.3 An earlier study also included crossing effects in the explanation of underlying mechanisms.9 These effects were hypothetically explained as due to corticocortical and spinal crossing fiber systems in a group of patients with cerebral infarction. Since we stimulated patients with spinal lesions, these effects might be better explained by spinal fiber systems than by influences on motor cortical systems. Examples for a crossing fiber system are the central pattern generator16,17,18,19 or the fibers of the flexor reflex afferents.18,20 Enhanced reactivity is often seen in patients with spinal cord lesions and spasticity. Therefore, such fiber systems could provide a new approach for a more detailed electrophysiological investigation to elucidate our findings as shown in earlier investigations.11
Some patients had an immediate decrease of spastic tone after repetitive stimulation. This might be due to a release of neurotransmitters after the unphysiological stimulation.9 The observed long-lasting decrease of spastic tone for about 20 h after stimulation cannot be attributed to neurotransmitter release, since neurotransmitters do not act for such a long time. The loss of supraspinal control leads to increased spinal reflexes below the lesion21 and therefore a disturbance of the physiological inhibitory and excitatory balance,22 that results in clinical spastic signs. They can be considered an attempt to regenerate spinal motor systems, suggesting the large potential for neuroplastic changes within injured spinal motor systems.23 These motor systems are influenced for short periods, that is, during physiotherapy3 or with treadmill training16 that leads to reduced spastic tone or automatic step cycling. We hypothesize that the repetitive magnetic stimulation can influence these injured spinal motor systems by reducing spastic tone. This reduction lasts for a limited time and might possibly reflect a short period of plastic change. However, more detailed investigations are clearly necessary to detect underlying pathophysiological mechanisms and to verify these hypothetical considerations.
Different motor thresholds of patients and normal subjects
The excitability of spinal motor neurons, as measured by the F-wave, is typically increased in patients with spastic tone increase.24,25 Therefore, one would expect that lower intensities are required to obtain a motor response, not higher ones as we observed in our study. Unfortunately, we currently have no explanation for this discrepancy.
Conclusion
The findings of a decrease in spastic tone after repetitive magnetic stimulation applied to lumbar nerve roots is of potential importance, because it may help to develop explanatory models and to extend the options available for the rehabilitation of spastic tone.
References
Lance JW . Symposium synopsis. In: Feldman RG, Young RR, Koella WP (eds). Spasticity: Disordered Motor Control. Year Book Medical Publishers: Chicago 1980, pp 485–494.
Sheean G . Clinical features of spasticity and the upper motor neurone syndrome. In: Sheean G (ed). Spasticity Rehabilitation, 1st edn. Churchill Communication Ltd 1998, pp 6–15.
Dietz V . Spastic movement disorder. Spinal Cord 2000; 38: 389–393.
Young RR . Spasticity: a review. Neurology 1994; 44 (Suppl 9): S12–S20.
Mauritz KH . Gait training in hemiplegia. Eur J Neurol 2002; 9 (Suppl 1): 23–29.
Bajd T, Gregoric M, Vodovnik L, Benko H . Electrical stimulation in treating spasticity resulting from spinal cord injury. Arch Phys Med Rehabil 1985; 66: 515–517.
Franek A, Turczynski B, Opara J . Treatment of spinal spasticity by electrical stimulation. J Biomed Eng 1988; 10: 266–270.
Hesse S, Jahnke MT, Luecke D, Mauritz KH . Short-term electrical stimulation enhances the effectiveness of botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett 1995; 201: 37–40.
Struppler A, Havel P, Müller-Barna P, Lorenzen HW . A new method for rehabilitation of central palsy of arm and hand by peripheral magnetic stimulation. Neurol Rehabil 1997; 3: 145–158.
Nielsen JF, Sinkjaer T, Jakobsen J . Treatment of spasticity with repetitive magnetic stimulation; a double-blind placebo controlled study. Mult Scler 1996; 2: 227–232.
Nielsen JF, Sinkjaer T . Long-lasting depression of soleus motoneurons excitability following repetitive magnetic stimuli of the spinal cord in multiple sclerosis patients. Mult Scler 1997; 3(1): 18–30.
Bohannon RW, Smith MB . Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987; 67: 206–207.
Bajd T, Vodovnik L . Pendulum testing of spasticity. J Biomed Eng 1984; 6: 9–16.
Wartenberg R . Pendulousness of the legs as a diagnostic test. Neurology 1951; 1: 18–24.
Maccabee PJ et al. Measurement of the electric field induced into inhomogeneous volume conductors by magnetic coils: application to human spinal neurogeometry. Electroencephalogr Clin Neurophysiol 1991; 81: 224–237.
Dietz V, Colombo G, Jensen L, Baumgartner L . Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol 1995; 37: 574–582.
Kjaerulff O, Kiehn O . Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J Neurosci 1997; 17: 9433–9447.
Quintern J, Straube A, Riener R, Roger C . Spinal mechanisms of the flexor reflex. Electroencephalogr Clin Neurophysiol 1996; 99: 392–398.
Rossignol S . Neural control of stereotypic limb movements. In: Rowell LB, Shepard JT (eds). Handbook of Physiology, Sect 12. Exercise: Regulation and Integration of Multiple Systems. American Physiological Society: Bethesda, MD 1996, pp 173–216.
Hultborn H, Conway BA, Gossard JP, Brownstone R, Fedirchuk B, Schomburg ED . How do we approach the locomotor network in the mammalian spinal cord? Ann N Y Acad Sci 1998; 860: 70–82.
Dietz V . Supraspinal pathways and the development of muscle-tone dysregulation. Dev Med Child Neurol 1999; 41: 708–715.
Bahr M, Bonhoeffer F . Perspectives on axonal regeneration in the mammalian CNS. Trends Neurosci 1994; 17: 473–479.
de Leon RD, Roy RR, Edgerton VR . Is the recovery of stepping following spinal cord injury mediated by modifying existing neural pathways or by generating new pathways? A perspective. Phys Ther 2001; 81: 1904–1911.
Fierro B, Raimondo D, Modica A . Analysis of F response in upper motoneurone lesions. Acta Neurol Scand 1990; 82: 329–334.
Schiller HH, Stalberg E . F responses studied with single fibre EMG in normal subjects and spastic patients. J Neurol Neurosurg Psychiatry 1978; 41: 45–53.
Acknowledgements
We thank Ms J Benson for editing the manuscript and Dr K Werhahn for help with the first recordings.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krause, P., Edrich, T. & Straube, A. Lumbar repetitive magnetic stimulation reduces spastic tone increase of the lower limbs. Spinal Cord 42, 67–72 (2004). https://doi.org/10.1038/sj.sc.3101564
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101564
Keywords
This article is cited by
-
Efficacy of Repetitive Trans-spinal Magnetic Stimulation for Patients with Parkinson's Disease: a Randomised Controlled Trial
Neurotherapeutics (2022)
-
Now is the Critical Time for Engineered Neuroplasticity
Neurotherapeutics (2018)
-
The physiological basis of neurorehabilitation - locomotor training after spinal cord injury
Journal of NeuroEngineering and Rehabilitation (2013)
-
Repetitive periphere Magnetstimulation
Der Nervenarzt (2009)