Abstract
We describe the case of a 47-year-old female who sustained a C5/6 fracture with C6 complete spinal cord injury 26 years ago. She presented with increased spasticity of the lower extremities, the abdominal wall and episodes of autonomic dysreflexia. Imaging of the spine revealed post-traumatic kyphosis at the level of the injury and degenerative changes of the lumbar spine with marked facet joint hypertrophy at the level of L4/5 causing severe spinal canal stenosis. Discussants of this case comment on the possible pathophysiological mechanisms causing autonomic dysreflexia, especially the development of degenerative changes, Charcot arthropathy and the role of tethering mechanisms. The diagnostic options and management approaches are also discussed.
Similar content being viewed by others
Case presentation
The patient is a 47-year-old female who sustained a C5/6 fracture with C6 complete tetraplegia 26 years ago. She is well adjusted socially and has a regular job.
At this time, she presented with increased spasticity of the lower extremities, the abdominal wall and episodes of autonomic dysreflexia. The symptoms developed 1½ years ago, with no trauma or acute disease at the time. The frequency and intensity of the symptoms has increased and can be triggered by trunk motions, especially leaning forward or backwards with activities such as dressing.
Further medical history reveals that since discharge from primary rehabilitation, the patient maintains a balanced pattern of voiding by triggering the abdominal wall. Urinary tract infections are rare, with none in the last 2 years. Bowel movements are well controlled, with no evidence of gastrointestinal problems. There are no skin lacerations or gross deformities of the extremities or the spine.
The patient denies any change in neurologic status other than the increase of spasticity. Clinical examination reveals a cervical 7 motor and sensory complete tetraplegia (ASIA A). Reflexes of the lower extremities (knee and ankle jerks) are markedly increased. It is not possible to determine any change in the neurological examination because the last documented examination took place more than 15 years ago.
Findings include marked atrophy of the paraspinal muscles, with trunk control in the sitting position maintained by the arms. There is bilateral decrease in the range of movement (ROM) of the hips (flexion/extension 80–0–0°/adduction-abduction 20–0–20°). Passive motion of the lower extremities induces mild extension spasticity. Straight leg raising past 30° induces more severe spasticity bilaterally and the patient explains that this maneuver provokes spasms and sweating. Inclination of the head, or lifting it off the bed does not have a similar effect.
The patient has had pelvic and lumbar spine films in AP and lateral views, which show early degenerative changes of both hips and marked degenerative changes of the lumbar spine. There is also hypertrophy of the facet joints L4/5. A bone scan shows increased uptake in the lower lumbar facet joints.
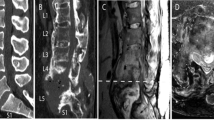
Urological consultation and workup was negative. An MRI study showed the old fracture of the cervical spine (Figure 1 and Figure 2 ) and severe, rather isolated, stenosis of the spinal canal L4/5 (Figure 4). There was no evidence of syringomyelia. Lateral views of the lumbar spine in flexion and extension are shown in Figure 3.
MRI of the thoracic and lumbar spine. The sagittal plane (a) demonstrates a normal lumbar spinal canal with the exception of the level L4/5 where almost complete stenosis is present. The axial plane sequence at this level (c and d) confirms this finding and shows massive hypertrophy of the facet joints as major source of the obstruction. The configuration of the spinal canal at the adjoining levels above (b) and below (e) is normal
Questions and responses
The following series of questions concerning the necessary diagnostic proceedings, differential diagnostic possibilities and management approaches was posed to the panel of experts. Edited responses are presented below, attributed by the names of the respondents.
-
1)
Based on the history and findings, would you agree to concentrate on the degenerative changes of the lumbar spine or would you want to rule out other pathologies first? Which other possibilities should be investigated and how should they be investigated?
-
2)
According to your experience, would you rate the isolated finding of this degenerative stenosis of the lumbar spinal canal as atypical, occasional or frequent in patients with a long history of paraplegia?
-
3)
Is the increase of spasticity consistent with the hypothesis that the stenosis of the lumbar spinal canal causes impingement of the nerves at the level L4/5 in this case? Should additional nerve injury at this level not result in a reduction of muscle tone?
-
4)
Why did the patient develop the changes in the lumbar spine? Would you think that lumbar ‘spinal instability’ is a problem in this case – especially given the inclination/reclination films?
-
5)
Before you decide what to do, which additional tests (eg EMG, test-brace, etc) would you want and how would the results change your treatment?
-
6)
What treatment would you propose?
-
7)
Are you aware of any ‘landmark’ literature about secondary degenerative changes to the spine in the case of long-standing paraplegia?
Comments: Humberto A Cerrel Bazo
In a nondisabled individual with stenosis of the spinal canal, we will probably find a positive straight leg raising test, pain and most probably signs of severe compression (eg muscle atrophy and loss of sensation). In a spinal cord injury (SCI), some of these classical symptoms are obscured by the effects of paralysis.
Spasticity in SCI patients is a typical sign of an intact second motor neuron. When increased or exacerbated, it may be because of an irritated spine or any cause of nociceptive stimulus. The clinical finding that reflexes of the lower extremities are markedly increased is not of great value because we cannot compare this with earlier findings. Here, these symptoms increase with bending the trunk forward, and even signs of autonomic dysreflexia are produced. Straight leg raising past 30° induces more severe spasticity bilaterally, and the patient explains that this maneuver provokes spasms and sweating. This sign may reflect or correlate with a positive straight-leg-raising test.
Whatever is happening in the spine is creating increased pressure in the spinal canal. Lumbar spine films show marked degenerative changes and hypertrophy of the facet joints of L4/5. The MRI confirms significant stenosis in the sagittal and axial views. The positive bone scan probably reflects inflammatory osteoarthritic changes. A local infection is unlikely because other no other signs of infection are present.
I do see degenerative changes of the lumbar spine with these findings occasionally.
In this case, it is necessary to evaluate the stenosis of the lumbar spinal canal by neurophysiological studies. Electromyograms and nerve conduction velocity should be used to investigate the L2–S1 territories. There should be an EMG of the paravertebral muscles, which are probably atrophic because of compression of the roots at the involved level.
A spinal tap (simple and not expensive) could be used to confirm the stenosis (elevated protein content) and to rule out intraspinal infection. However, in this case the MRI findings are conclusive by themselves.
It might be necessary to re-evaluate the increased uptake in the bone scan in the lower lumbar facet joints. This could be done by a leucocyte bone scan. It is not a priority and may be considered later on.
Since there is stenosis of the canal, bending of the trunk and/or moving of the lower extremities may increase the endo-pressure of the spinal canal, creating signs of compression (autonomic dysreflexia, increased spasms). Obviously, the compression is not severe enough to produce signs of direct nerve compression. Only this would produce muscle atrophy and flaccid paralysis. This effect might be responsible for atrophy of paravertebral muscles. Again, any form of a cyst or syringomyelia in the more proximal spine has to be ruled out before settling on lumbar stenosis as the origin of the problem.
There are no signs for spinal instability on the films supplied. I only see a rigid spine.
As treatment, spinal decompression could be advisable. Stabilization of the spine should be performed if 50% or more of the facet joints are destroyed in this process.
Comments: Patrick J Kluger and Fahed Selmi
In the majority of cases, autonomic dysreflexia is associated with the disturbance of bowel or bladder function.1,2,3 This can be explained by the intense linkage of these organs to the autonomic nervous system. Apart from this, autonomic dysreflexia can be caused by a large variety of disorders, many more than the ingrown toe-nail traditionally mentioned. Intriguingly, disturbances of bowel or bladder function could be taken simply as presenting the most common disorder in chronic transverse lesions of the cervical and the upper thoracic region. To our knowledge, no statistical analysis was done to actually assess the prevalence of particular pathologies in causing autonomic dysreflexia.
To define the disorder, which individually causes dysreflexic episodes, the observation of trigger mechanisms is most important. If patients develop symptoms on movement and sitting up, as in the reported case, lesions of the musculoskeletal system's paralyzed sector should be considered. Rarely, kidney stones may trigger autonomic dysreflexia during sitting up as well, but this was ruled out in the reported patient.
We learned from the case report that a severe spinal stenosis at L4/5 is present in the patient, and that there are degenerative changes in both hips, restricting the hip flexion to 80°, bilaterally.
In Figure 2 (MRI study T2, lateral view midline) we see, apart from the described region of interest at the injury-related alterations at C6–T1, a blurred change in the bone signal of the vertebral body of T5, at the very bottom of the picture, which had not been addressed in the report. It would be easy to rule out the suspicion of a tumor at T5 by performing a CT scan or, simply, by centering down the MRI's region of interest. Then, the signal alterations will probably appear to be fatty accumulations, as present in the patient's lumbar spine (which could be confirmed in T2 with fat suppression).
Assuming that no pathology is present at T5, the hip joints and the lumbar spine remain under suspicion.
Spinal disorders below the level of the transverse lesion are known to possibly cause autonomic dysreflexia,4 and from our institution two cases of spinal Charcot joints were reported, which caused autonomic dysreflexia.5
Degenerative changes of the lumbar spine are frequent in wheelchair users, but they will often show no clinical symptoms, and so remain undetected. Considering that X-rays in the AP view will disclose quite a substantial degeneration, a look at the routinely performed abdominal X-rays for urological checkups allows an estimation of the incidence. To our knowledge, this has not yet been investigated. The degenerative changes are caused by malaligned spinal statics and by the SCI person's excessive use of the lumbar mobility, without sensitive protection. Spinal Charcot joints are more easily detected, and they are well known as ‘neuropathic spinal arthropathy’.6,7,8,9,10 Pathophysiologically, we understand this entity as the organism's occasional failure in its usual response to the segment's mechanical overload by immobilizing bony apposition, which leads to pathologic hypermobility and, eventually, to segmental destruction. Its occurrence in paraplegics is another hint of the frequency of degenerative lumbar disorders in these patients.
The paraclinical, neurological investigation of lumbar spinal stenosis, in our experience, does not show very significant results. The neurology of the lower limbs in the reported patient should show a similar, and not very conclusive, pattern of mixed pre- and postnuclear alterations, as is found in the frequent coincidence of cervical and lumbar spinal stenosis, in ambulatory patients. Therefore, the unaltered pattern of reflex bladder voiding, as was found in the urological workup, in our opinion, does not necessarily contradict the idea of the spinal stenosis causing the autonomic dysreflexia. A study of spinally evoked motor potentials in the reported case would be of scientific interest, as well as a treadmill test that could shed some light on the discussion about a spinal center for motor coordination in humans — one carrying a risk of hardly controllable autonomic dysreflexia, and the other being technically difficult to undertake in the described patient. We do not recommend both. The crucial distinction between hip versus spine responsibility for autonomic dysreflexia in this particular patient should be found clinically.
The ROM of both hips is reported to be restricted to 80–0–0° for flexion/extension. Considering the significant loss of the lumbar profile in this patient (Figure 3), the ROM's restriction (assessing hip arthropathy clinically, the ROM for internal/external rotation should not be omitted) can be taken as more concentric than the given values indicate. This fits better into the typical pattern of a degenerative arthropathy in the hip joints. If this disorder would form the cause for autonomic dysreflexia in the described case, auto-nomic dysreflexia symptoms should be provoked by the usual clinical tests for hip arthropathy (eg maximal flexion+internal rotation, compression), the onset of symptoms should happen immediately, and there should be no difference in symptoms between the passive straight-leg raising and the passive hip flexion with flexed knees.
If the massive spinal stenosis at L4/5 (Figure 4) causes the patient's autonomic dysreflexia, the anticipated symptoms should have a more crescendoing character, and they should occur under verticalization on a tilt table as well (which opens the interesting discussion as to what extent actually a spinal claudication is caused mainly by an increase of blood pressure in the peridural venous system rather than by activity-related general swelling).
Further assurance in excluding the hips from causing autonomic dysreflexia can be obtained by performing activities known to provoke autonomic dysreflexia, after instillating a local anesthetic into both hip joints.
If, as we may expect, the suggested tests (standing on a tilting table and local anesthesia of both hips during provoking activities) confirm spinal stenosis as being the cause for autonomic dysreflexia in the reported patient, spinal decompression is the therapy of choice. According to the imaging (Figure 4), it may be difficult to decompress L4/5 without substantially weakening the segment's stability (see above about the mechanisms leading to neuropathic spinal arthropathy), in which case instrumentation and fusion have to be considered. If so, the appropriate segmental extent of this procedure and the segmental alignment, for which the instrumentation should strive, is put under question.
The MRI (Figure 4) does not show L5/S1, and this segment is not commented on in the report, but the plain films (Figure 3) show quite a marked degeneration of L5/S1. A degenerative spondylolisthesis, a preserved mobility, and a vacuum phenomenon as a sign of degenerative changes in the disc can be seen. This segment's future would surely be negatively affected by fixing L4/5 exclusively, and if fixation of L4/5 is felt to be necessary, the lumbo-sacral junction should be included.
In defining the appropriate alignment, which an instrumentation and fusion should establish, the restricted ROM of the hip joints comes into concern again. Generally, one would like to restore the normal sagittal profile of the lumbar spine in order to improve the vertebral column's elastic capacities. Before doing so, adverse effects caused by the restricted hip flexion should be considered as well as the given mobility of the upper lumbar spine. In the flexion/extension X-rays (Figure 3), quite a poor lumbar mobility is demonstrated. While the MRI (Figure 4) indicates massive degenerative immobilization of the lower thoracic and of the thoraco-lumbar region, not much of this is present in the upper lumbar segments, which is pictured as hardly affected in the plain X-rays as well. The impressive stiffening of the same region in flexion/extension (Figure 3) may be understood, therefore, as a flat-back, reacting to the pathology L4/5 and possibly lumbo-sacral. The spontaneous lumbar alignment under anesthesia, in the prone position on the theater table, will answer the question.
If, surprisingly, the recommended tests would prove the hip joints as causing the autonomic dysreflexia, we would regard this as one of the very rare indications to implant a total hip prosthesis in an SCI patient, or alternatively to perform a Girdlestone arthroplasty.
Comments: Thomas Meiners
Further procedures on grounds of history and findings
The results of the clinical examination and the imaging investigations combined yield the following picture:
-
There is increasing spasticity of the lower extremities with autonomic dysreflexia triggered by movement of the trunk and by the Lasègue maneuver.
-
Motor, sensory and vegetative neurology findings are reported as unchanged, reflexes are maintained and there are no signs of additional peripheral neurological defects.
-
Kyphosis also involving the cervical spine is present and there is possible tethering of the cervical spinal cord.
-
The lateral X-ray films (inclination, reclination) reveal irregular delimitation of vertebrae L4, L5 and S1, and also sclerosis of the end-plates close to the intervertebral discs at levels L4/5 and L5/S1, the MRI discloses a change in the signal from the intervertebral disc at L5/5, and considerable hypertrophy of the vertebral joints with segmental stenosis at L4/5.
It will be necessary to find out whether the increasing spasticity and autonomic dysreflexia have arisen through progressive post-traumatic myelomalacic myelopathy11,12 or through bony changes in the lower lumbar spine with possible neural impingement.
The extent of tethering of the cervical and thoraco-lumbar spinal cord can be investigated by kinematic MRI to test the mobility of the spinal cord.13,14
The spinal stenosis at L4/5 with concomitant stenosis of the corresponding intervertebral foramina by the hypertrophic joint facets revealed by MRI affects the L5 roots the most. An EMG investigation should be performed to elucidate whether there is peripheral denervation of the muscles associated with L5 (tibialis anterior and extensor hallucis longus muscles). The pronounced sclerosis of the vertebral body end-plates at L4, L5 and S1, the diminution of the disc spaces L4/5 and L5/S1, and the hypertrophy of the facet joints can also be interpreted as incipient neuroarthropathy of the spine. The changed signal from L4/5 on MRI also suggests this. Oblique films of the lumbar spine and complete imaging of the lower lumbar spine by MRI will perhaps yield further relevant information.
A similar case of neuropathic osteoarthropathy of the spine with autonomic dysreflexia has been reported in the literature very recently.15
Frequency of such findings in patients with long-term history of paraplegia
The changes in the lower lumbar spine are a sign of incipient neurogenic osteoarthropathy of the spine. These changes are rare.9,16 I recall seeing only five such patients. I cannot recollect any typical degenerative patterns in the spine from my own experience. Bhate et al17 found mainly sacro-iliac changes (42%) among 200 patients with chronic spinal cord legions, and syndesmophytes, osteophytes and calcification in only a few cases (12%). Park et al18 saw 200 patients with chronic spinal cord syndrome and found osteophytes, paraspinal ossification and syndesmophytes in 43% of the tetraplegic patients and mainly osteophytes in 44% of the paraplegic patients in this series.
Increase of spasticity and impingement
The increasing spasticity can be caused by alterations to the intervertebral discs and the bony changes at L5/S1 and L4/L5. The spinal stenosis does not necessarily cause a peripheral paralysis of the roots at the level of L5. Nonetheless, a careful investigation should be made by means of EMG to check for peripheral neuropathies, with special reference to the muscles of root L5.
Reasons why precisely the lumbar region is involved
According to the literature, the lumbar spine is the predilection site for neurogenic osteoarthropathy of the spinal column. The inclination and reclination films of the lumbar spine do not reveal any hypermobility. The sclerosis of the end-plates of L4, L5 and S1, the reduced thickness of the intervertebral discs L4/5 and L5/S1 and the hypertrophy of the joint facets suggest segmental instability, however, mainly of segment L4/5.
Further tests and implications of their results for treatment
Dynamic MRI measurements may lead to the assumption that the mobility of the spinal cord has changed in both the proximal and the distal segments. Further examination under lumbar spinal anesthesia could help. If no signs of autonomic dysreflexia were triggered by the Lasègue maneuver under this condition, this would indicate a lumbar origin of the symptoms. If no change would be appreciated, it means that the post-traumatic myomalacic myelopathy with the cervical tethering is responsible for the situation.
Proposed treatment
In the case of post-traumatic progressive myomalacic myelopathy, laminectomy C5 and C6, untethering and duraplasty are indicated. For neurogenic osteoarthropathy of the lumbar spine, there would be a choice of conservative or surgical management. An individually made corset immobilizing the lumbar spine, 6-monthly X-ray and MRI follow-up, clinical and neurological follow-up examination with urodynamic testing and pressure measurement of sphincter ani externus muscle and EMG follow-up to exclude peripheral neuropathic alterations would be the necessary measures with a conservative approach. Otherwise surgical management should include anterior and posterior stabilization of L4/5 with spinal canal revision followed by a brace for 6 months.
Comments: Alexander Vaccaro and John Ditunno
The patient presented in this case report is a 47-year-old female who incurred a flexion compression injury to the C5/6 vertebral body at 21 years of age. As a result of that accident she incurred a complete C6/7 ASIA A SCI. She adjusted well neurologically to this accident up until approximately 1½ years ago when she began to develop symptoms of autonomic dysreflexia, and increased spasticity of her lower extremities and abdominal musculature. An MRI of her cervical spine revealed an approximately 40° kyphotic deformity between C4 and C7 with evidence of spinal cord atrophy in this region with no obvious syringomyelia. The MRI of her lumbar spine revealed evidence of marked central, lateral recess and foraminal stenosis at the L4/5 level in the setting of severe facet hypertrophy and the presence of a synovial cyst.
With regard to the question ‘Could the degenerative changes to the lumbar spine account for the neurologic changes illustrated by the patient?’, the answer is unclear. Further neurological deterioration from spinal stenosis should result in decreased reflexes rather than hyper-reflexia, so that this possibility is unlikely. Autonomic hyper-reflexia, however, may be the result of any irritable focus and there is a case report of a ‘sacral insufficiency stress fracture’ in a person 25 years postinjury with a complete C5 injury.4 It is well known that a small percentage of patients with central nervous system dysfunction are at risk for a destructive musculoskeletal condition known as a Charcot spine. This is essentially a neuropathic pseudo-joint that develops as a response to repetitive loading to tissue that is deprived of the protective neural feedback systems such as afferent somatic sensory innervation or proprioception. Symptoms of this disorder may include regional muscular spasticity and symptoms of autonomic dysreflexia. Patients with a Charcot spine may complain of phantom back pain associated with spasticity with spinal motion. Surgical stabilization of this deformity often lessens a patient's symptoms. I am aware of no cases where advance degenerative disease triggered a similar neuropathic feedback mechanism increasing muscular spasticity and symptoms of autonomic dysreflexia. It is well known that patients with high SCI over time develop evidence of disuse osteoporosis of their lower extremities as well as adaptive and reactive changes of degeneration in their axial skeleton. This has been reported by several researchers. Szollar et al19 evaluated the demineralization response in tetraplegic and paraplegic men over time and showed that SCI was associated with bone loss post-traumatically in the femoral region of young men, although this loss did not begin until approximately 1-year postinjury. It was more pronounced in the 20–29-year-old age group. A surprising finding, however, was that the lumbar bone mass was stable and actually increased with age, regardless of the age at the time of injury. The authors explain this as a result of increased bone density, most likely because of degenerative changes that occur owing to the excessive loads experienced by the axial skeleton not protected by a normal paraspinal muscular system.
The question is: ‘can such significant degenerative changes incite the neurologic instability typically seen with a Charcot neuropathic joint’. Before ascribing the patient's symptoms to this etiology, it is imperative that other causes of excessive stimulation to the central nervous system be ruled out, that is, tethering of the spinal cord at the level of the patient's cervical kyphosis with further ascension of spinal cord atrophy. This may be considered less likely in this case by the fact that the patient continues to function as a C7 ASIA A and has not manifested any change in upper extremity weakness. A progressive neurological deficit may or may not occur with a tethered cord that is producing autonomic dysreflexia. In a reported series of autonomic dysreflexia associated with tethered cord and post-traumatic cystic myelopathy (ruled out by MRI in this case), the symptoms of dysreflexia can occur alone or associated with neurological deficit.11 Also, serial plain X-rays of the cervical spine should be analyzed to rule out the gradual increase in her cervical kyphotic deformity. Additionally, tethering of the neuroelements (conus medullaris and cauda equina) should be excluded as a cause of neurologic dysfunction. This can be investigated with an MRI with gadolinium of the terminal spinal cord.
The findings of degenerative stenosis in the lumbar region should be considered a typical finding in the aging SCI patient, especially if the injury occurred during youth. A possible cause of neural tethering in this patient may be entrapment of the spinal nerve roots at the level of lumbar stenosis (L4/5). This may be simulating a typical tethering phenomenon causing stress on the central nervous system, resulting in the symptoms of autonomic dysreflexia and muscular spasm. Surgical intervention designed to decompress the neural elements and stabilize the involved motion segments may indirectly untether the spinal cord, lessening the patient's symptomatology. An interesting test may be to subject the patient to a trial of bracing to see if lessening the excursion of the lumbar spine decreases the patient's symptoms of spasticity. If so, then one may consider the possibility of surgical intervention. If the patient's symptoms are unaffected, I would be extremely hesitant to propose any type of surgical intervention in this setting. To date, there is no literature supporting the causative relation between lumbar spinal degenerative changes and increasing spasticity in the setting of tetraplegia.
Treatment provided
After completing the diagnostic workup as demonstrated we decided that the only pathology we could clearly identify was the stenosis of the spinal canal at the level of L4/5. The positive reproduction of the problem by the straight leg raising test pointed towards the lumbar spine as the origin of the problem. We considered a tethering mechanism but felt that such a condition would have to be explained by the lumbar pathology. We did not expect any major segmental instability because of the rather normal findings of the lateral flexion and extension films. Further tests like EMG were omitted because we did not expect a clear-cut finding that would change our treatment decision.
We explained the situation to the patient and recommended a surgical decompression with the possibility of an instrumented posterolateral fusion if adequate decompression should result in facet joint injury. The patient gave her consent and urged for rapid intervention because the symptoms were experienced as quite intolerable.
The surgical procedure was performed using a posterior midline incision. After stripping of the paraspinal muscles from the spinous processes and the lamina, we found grossly enlarged facet joints L4/5 bilaterally. A segmental motion test using clamps applied to the spinous processes showed marked hypermobility of the L4/5 segment compared to the mobility of the adjoining segments.
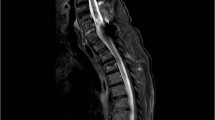
Bilateral hemilaminotomy was performed at this level, leaving the spinous process and the posterior spinous ligament in place.20 The enlarged medial aspect of the facet joints was removed together with thick capsular layers encroaching the spinal canal. After demonstrating free passage of a probe to the roots and into the spinal canal cranially and caudally, we decided to perform an instrumented posterior–lateral fusion to treat the hypermobility of the segment. The result is demonstrated in Figure 5.
However, in regard to the discussion of a tethering mechanism, there was no adhesion of the dura to the site of the stenosis nor was there any indication that intradural structures were adherent to the dura. After decompression the dura expanded in a normal fashion.
For about 2 weeks after surgery, the patient had some increase in general spasticity but it could no longer be provoked by straight-leg raising. The symptoms gradually subsided and on discharge the patient stated that all symptoms had disappeared.
There is now a 2-year follow-up, and no recurrence of any symptoms has been noted. The instrumentation is still intact.
Discussion
All the authors of the comments to the case stated above basically agree that the changes of the lumbar spine may account for the patient's problem after exclusion of other pathologies that are known to trigger autonomic dysreflexia. The interesting differences lie in the possible differential diagnosis suggested and the approach to the differential diagnosis.
We never really considered tethering (of cervical or lumbar origin) as a possibility. Tethering of the spinal cord as a source of secondary neurologic deterioration has for a long time been associated mainly with meningomyelocele. One of the first reports of tethering as a problem in adults after SCI has been given by Ragnarsson et al.21 It has gained more attention lately and the literature indicates that it is accepted as a source of problems by a mechanism that is caused by adhesions at the level of the injury.22,23,24 However, there is still no uniform definition of tethering or of the diagnostic approach.25,26
To include cervical tethering in our list of differential diagnosis, we would have expected changes to the neurologic status of the upper extremities and we would not be able to explain why the straight leg raising test so constantly reproduced the symptoms. However, we agree that the MRI of the cervical spine suggests the possibility of cervical tethering, and the findings are quite comparable to the examples given in the paper of Lee et al.11
Lumbar tethering also was not on our list of problems. First, we are not aware of any report that names tethering of the spinal cord as a cause of neurological deterioration in the vast population of patients with massive stenosis of the spinal canal without previous SCI or after lumbar disc surgery with scarring. Lumbar tethering is usually associated with a malformation or intraspinal lipoma, but there is the definite possibility that this point might warrant future research. In this case, we feel that in hindsight it is probably even less likely because there were no adhesions to the dura and the dura did not show any signs of adherent intradural structures.
Consistent with the opinions expressed, we decided that the pathology must originate from the segments L4/5 with the stenosis of the spinal canal caused by some mechanism owing to the unphysiological loading over a long period of time (like a Charcot joint). However, we failed to appreciate the instability of this segment preoperatively. We felt that this might have been an ‘unstable segment’, but that this problem had been solved by the natural course of degeneration of the spinal segments with reduced motion as an end stage of disease. The flexion – extension films seemed to support this notion. This was also the reason why we did not carry out any bracing tests.
We will follow the patient closely because we are also worried that the fusion of one segment will place the adjacent segments at risk. However, we felt that we would gain time by trying to preserve motion segments now. Also it might be necessary to enlarge the fusion to the sacrum later.
We consider this case as rather unusual but think that the community of SCI therapists will be confronted with similar problems more often, as long-term survival of an SCI is now the rule, not the exception. The ideas of diagnostic approach given above will certainly influence our own approach. Finally, we look forward to see more focus on issues like degenerative changes of the lumbar spine after SCI, Charcot arthropathies and secondary tethering of the spinal cord.
References
Karlsson AK . Autonomic dysreflexia. Spinal Cord 1999; 37: 383–391.
Mathias CJ, Bannister R . Autonomic disturbances in spinal cord lesions. Autonomic failure. In: Mathias CJ, Bannister R (eds). A Textbook of Clinical Disorders of the Autonomic Nervous System. Oxford: Oxford University Press, 1999, pp 494–513.
Mathias CJ, Frankel HL, Cole JD . Management of cardiovascular abnormalities caused by autonomic dysfunction in spinal cord injury. In: Illis LS (ed). Spinal Cord Dysfunction. Oxford: Oxford University Press, 1992, pp 101–120.
Beard JP, Wade WH, Barber DB . Sacral insufficiency stress fracture as etiology of positional autonomic dysreflexia: case report. Paraplegia 1996; 34: 173–175.
Selmi F, Frankel HL, Ponnuswamy K, Apostopoulos V . Charcot joint of the spine, a cause of autonomic dysreflexia in spinal cord injured patients. Spinal Cord, in press.
Kalen V, Isono SS, Cho CS, Perkash I . Charcot arthropathy of the spine in long-standing paraplegia. Spine 1987; 12: 42–47.
Schwartz HS . Traumatic Charcot spine. J Spinal Disord 1990; 3: 269–275.
Slabaugh PB, Smith TK . Neuropathic spine after spinal cord injury. A case report. J Bone Joint Surg Am 1978; 60: 1005–1006.
Sobel JW, Bohlman HH, Freehafer AA . Charcot's arthropathy of the spine following spinal cord injury. A report of five cases. J Bone Joint Surg Am 1985; 67: 771–776.
Wirth CR, Jacobs RL, Rolander SD . Neuropathic spinal arthropathy. A review of the Charcot spine. Spine 1980; 5: 558–567.
Lee TT et al. Progressive posttraumatic myelomalacic myelopathy: treatment with untethering and expansive duraplasty. J Neurosurg 1997; 86: 624–628.
Edgar R, Quail P . Progressive post-traumatic cystic and non-cystic myelopathy. Br J Neurosurg 1994; 8: 7–22.
Levy LM . MR imaging of cerebrospinal fluid flow and spinal cord motion in neurologic disorders of the spine. Magn Reson Imaging Clin North Am 1999; 7: 573–587.
Muhle C et al. Kinematic MR imaging in surgical management of cervical disc disease, spondylosis and spondylotic myelopathy. Acta Radiol 1999; 40: 146–153.
Thumbikat P, Ravichandran G, McClelland MR . Neuropathic lumbar spondylolisthesis–a rare trigger for posture induced autonomic dysreflexia. Spinal Cord 2001; 39: 564–567.
Park YH, Taylor JA, Szollar SM, Resnick D . Imaging findings in spinal neuroarthropathy. Spine 1994; 19: 1499–1504.
Bhate DV, Pizarro AJ, Seitam A, Mak EB . Axial skeletal changes in paraplegics. Radiology 1979; 133: 55–58.
Park YH et al. Patterns of vertebral ossification and pelvic abnormalities in paralysis: a study of 200 patients. Radiology 1993; 188: 561–565.
Szollar SM et al. Demineralization in tetraplegic and paraplegic man over time. Spinal Cord 1997; 35: 223–228.
Benz RJ, Garfin SR . Current techniques of decompression of the lumbar spine. Clin Orthop 2001; 384: 75–81.
Ragnarsson TS, Durward QJ, Nordgren RE . Spinal cord tethering after traumatic paraplegia with late neurological deterioration. J Neurosurg 1986; 64: 397–401.
Silber JS, Vaccaro AR, Green B . Summary statement: chronic long-term sequelae after spinal cord injury: post-traumatic spinal deformity and post-traumatic myelopathy associated with syringomyelia. Spine 2001; 26(Suppl): S128.
Lee TT, Alameda GJ, Camilo E, Green BA . Surgical treatment of post-traumatic myelopathy associated with syringomyelia. Spine 2001; 26(Suppl): S119–S127.
Vaccaro AR, Silber JS . Post-traumatic spinal deformity. Spine 2001; 26(Suppl): S111–S118.
Huttmann S et al. Surgical management of tethered spinal cord in adults: report of 54 cases. J Neurosurg 2001; 95 (Suppl): 173–178.
Witkamp TD et al. Medullary cone movement in subjects with a normal spinal cord and in patients with a tethered spinal cord. Radiology 2001; 220: 208–212.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abel, R., Cerrel Bazo, H., Kluger, P. et al. Management of degenerative changes and stenosis of the lumbar spinal canal secondary to cervical spinal cord injury. Spinal Cord 41, 211–219 (2003). https://doi.org/10.1038/sj.sc.3101435
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101435
Keywords
This article is cited by
-
Case series on the Charcot neuroarthropathy in hands after cervical central cord syndrome
BMC Musculoskeletal Disorders (2022)