Abstract
Study design: Analysis of the sequential waveform changes of the spinal-cord-evoked potentials (SCEPs) associated with progressive cord compression in the cat.
Objectives: To document the phenomenon of paradoxical enhancement of SCEPs despite conduction abnormalities and to evaluate its possible significance.
Setting: Kochi Medical School, Kochi, Japan.
Methods: SCEPs were recorded simultaneously at four serial intervertebral levels, from T6–7 to T9–10 caudal to, and at three serial levels from T2–3 to T4–5 rostral to the compression site at T5–6 following epidural stimulation at L6 in 14 cats.
Results: Caudal to the compression site, the area of negative peak significantly increased toward maximal values of 277±36 (mean±SE), 151±9 and 110±4% as compared to the baseline precompression values (100%) at T6–7, T7–8, and T8–9, respectively. Rostral to the compression site, the area of negative peak significantly increased before subsequent deterioration and reached 105±2, 106±2, and 104±2% at T4–5, T3–4, and T2–3, respectively. The onset of negative peak enhancement, recorded either caudal or rostral to the compression site, showed a close temporal correlation (r>0.8, P<0.001) with that of the prolongation in latency of SCEPs at T2–3.
Conclusions: A progressive focal conduction block induced by compression of the spinal cord can paradoxically enhance the ascending SCEPs both caudally and, though less consistently, rostrally, representing a warning of the impending risk of paraplegia.
Similar content being viewed by others
Introduction
The arrest of impulse propagation along intact axons, also known as a conduction block, is a physiological consequence of spinal cord compression. This phenomenon is of considerable importance, because it is an indication of a potentially treatable paraplegia and tetraplegia.1 Analysis of spinal-cord-evoked potentials (SCEPs) is used as a tool to detect conduction block during surgical monitoring2,3 and to determine the level of spinal cord responsible for main functional changes.4,5,6,7,8,9 Employing this technique, the reduction in size of the recorded response is interpreted as conduction block, while the increase in size of response is considered as its resolution. Computer modelling, however, disputed the validity of this interpretation predicting that partial conduction block could cause an increase in the size of SCEPs instead.10,11 Furthermore, an animal experiment employing constant-speed progressive cord compression showed a transient increase in the amplitude of ascending SCEPs at the compression site.12 Therefore, an enhancement of SCEPs should be interpreted with caution, since it may not necessarily imply functional recovery. To gain further insight into this problem, we studied sequential waveform changes of SCEPs recorded simultaneously from seven sites along the spinal cord during progressive cord compression in the cat.
Methods
Animal preparation
A total of 14 adult cats weighing 2300–5660 g were investigated. The animals were initially anesthetized with intraperitoneally injected pentobarbiturate (35 mg/kg body weight) followed by halothane after intubation and connection to an automatic ventilator. Partial laminectomies and removal of ligamentum flavum were performed to allow sufficient access to epidural space at levels from T2–3 to T9–10 and at L6–7. During recording sessions, animals were immobilized with intramuscular injections of vecronium bromide (0.25 mg/kg). In some cases, small additional amounts of vecronium bromide were injected when needed. A pool of warm paraffin oil was used to cover the wound. Its temperature was maintained steady between 36 and 38°C throughout the whole experiment.
The study was approved by the Committee for Animal Experimentation Ethics of the Kochi Medical School.
Cord compression
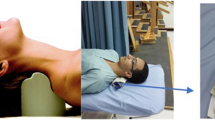
A specially designed external compression device was rigidly fixed to T5 and T6 spinous processes (Figure 1). The device incorporated a vertically mounted brass screw with a 5 mm diameter plastic plate loosely attached to the end. The screw was advanced to ensure progressive compression of the surface of unopened dura mater at T5–6. Compression was increased every 2 min by turning the screw through 90° and eventually producing an advance of 0.1 mm. The final level of compression was defined at the point where the negative peak of the SCEP recorded rostrally to the compression site had been abolished.
Schematic drawing of the general experimental design. Epidural stimulation at L6 was performed during progressive cord compression at T5–6 level in the cat. Ascending SCEPs were recorded simultaneously at seven sites above and below. The ‘0’ level represents the site of compression (T5–6). Other levels are numbered in order of increasing distance from the ‘0’ level. The minus sign indicates caudal direction
Stimulation
The spinal cord was stimulated using a pair of electrodes (UKG-100-2PM, Unique Medical Corp., Tokyo, Japan) with two platinum tips at the end of an 18 gauge polyethylene tube.2,13,14 Electrodes were introduced into the lateral epidural space under L6 lamina (see Discussion). Electrical stimulation consisted of square waves of 0.1 ms duration delivered at a rate of 3/s.
Recordings
An eight-channel amplifier (Dantec Evomatic 8000, Dantec Medical, Skovlunde, Denmark) allowed simultaneous recording of SCEPs at four serial intervertebral levels from T6–7 to T9–10 caudal to, and at three serial levels from T2–3 to T4–5 rostral to the compression site at T5–6. At each recording site, a silver ball electrode of 1 mm diameter (UL-3010 No64, Unique Medical, Tokyo, Japan) was placed in the epidural space ipsilaterally to the side of stimulation and served as an active electrode (G1). As a reference electrode (G2), a series of needles (Dantec 13R23) were inserted into the erector spinae muscles at the same level as G1. A pair of alligator clips was attached to the skin at the operative site as a ground electrode. The ‘0’ level represented the site of compression (T5–6). Other levels were numbered in order of increasing distance from the 0 level, assigning the minus sign caudally (Figure 1).
Precompression recordings were performed to establish the baseline value that was equated to 100%. Then, SCEPs were recorded after every additional step of compression. Each test set comprised an average of 100 summated potentials with a frequency response from 100 Hz to 5 kHz. Positive and negative components of SCEPs were measured separately to delineate the characteristic waveform changes of each component, which followed a different time course after compression. Measurements of SCEPs included: (1) latencies from the stimulus artifact to the initial positive peaks, (2) amplitudes from the baseline to the initial positive and the major negative peaks, and (3) areas (voltage–time integral) of the initial positive and the subsequent negative phases (a bottom trace in Figure 2A).
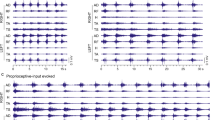
(A). Representative samples of ascending SCEPs recorded at four serial intervertebral levels (‘−1’, ‘−2’, ‘−3’, and ‘−4’) caudal to the compression site in a cat. Each potential represents the average of 100 measurements. SCEPs were recorded before compression (a), and with the advance of compression screw by 1.8 mm (b) and 2.3 mm (c). The N1 peak progressively increased in size at all levels except ‘−4’, compared to precompression recording (a). The N1 peak enhancement was accompanied by a progressively slower and prolonged downstroke of the peak (oblique arrows in (c)). The P1 peak remained nearly constant throughout the experiment. (B) Representative samples of ascending SCEPs recorded at three serial intervertebral levels (‘+1’, ‘+2’, and ‘+3’) rostral to the compression site in a cat. Each potential represents the average of 100 measurements. SCEPs were recorded before compression (a), and with the advance of compression screw by 1.8 mm (b) and 2.3 mm (c). The N1 peak initially increased in size (vertical arrows pointing up in (b)), and then progressively decreased toward the final recording (c). In contrast, the P1 peak progressively increased in the area at ‘+1’ and, conversely, showed a progressive reduction at ‘+2’ and ‘+3’
Data analyses
All serial data from 14 animals were aligned to the final compression level rather than to the beginning of compression, because the degree of compression required to change the SCEP varied from one animal to another as a result of variations of the spinal cord/spinal canal ration among the animals.
To test for a statistical difference in amplitude, area, and latency of a series of SCEPs, we used Friedman's test followed by the Wilcoxon t-test with Bonferroni correction. For correlation analyses, we used Spearman's rank correlation coefficients. Values are given as mean±SE and were considered significant at P<0.05.
Results
With G1 placed in the lateral epidural space, SCEPs consisted of a major triphasic wave (P1–N1–P2, Figure 2A) followed by smaller polyphasic waves. Recorded SCEPs were as follows.
SCEPs recorded caudally to the compression site
Compression altered the waveform of SCEPs caudally to the compression before the impulse crossed the compression site. With increasing degree of compression, the N1 peak progressively increased in amplitude and area toward maximal values of 199±14 and 277±36% (both P<0.01) at ‘−1’, 124±4 and 151±9% (both P<0.01) at ‘−2’, and 104±2 and 110± 4% (both P<0.05) at ‘−3’, compared to the precompression values (Figures 2A and 3A). At ‘−4’, the N1 peak did not show a significant enhancement both in amplitude (102±4%; P>0.3) and area (102±4%; P>0.06). As mentioned before, the degree of compression required to enlarge the N1 peak varied from one animal to another, presumably reflecting variations of the spinal cord/spinal canal ratio among the animals. Therefore, Figure 3 is presented with all graphs aligned to the final compression level (see legend). Interestingly, ‘the onset of N1 peak enhancement at ‘−1’, ‘−2’, and ‘−3’ levels showed a close temporal correlation (r=0.97 for amplitude; P<0.001, r=0.90 for area; P<0.001) with that of the prolongation in P1 latency of SCEPs recorded rostral to the compression (‘+3’ level).
(A) Changes in the mean relative amplitude (left) and area (middle) of the N1 peak and the mean latency changes of the P1 peak (right) of ascending SCEPs recorded at four serial intervertebral levels (‘−1’, ‘−2’, ‘−3’, and ‘−4’) caudal to the compression site during progressive cord compression in 14 cats. Abscissa shows the degree of cord compression as a result of a cumulative advance of the compression screw. Since all graphs are aligned to the final compression level, ‘0’ mm represents the final compression level with milder compression levels assigned a minus sign. Ordinate shows amplitude and area ratios, and latency change. Amplitude and area ratios are expressed as percentage of precompression values. Latency change is expressed in milliseconds. Bars indicate standard error (SE). The mean value statistically different from the baseline is marked with an asterisk (*). Note that there is a significant and progressive increase in N1 amplitude and area at ‘−1’, ‘−2’ and ‘−3’, but not at ‘−4’. The P1 latency shows no significant change throughout the whole experiment. (B) The same arrangement as in (A) to show changes of ascending SCEPs recorded at three serial intervertebral levels (‘+1’, ‘+2’, and ‘+3’) rostral to the compression site during progressive cord compression in 14 cats. With the stepwise increase of the compressive load, the N1 peak significantly increased in amplitude at ‘+1’ and ‘+2’, and in area at ‘+1’, ‘+2’, and ‘+3’, before subsequent deterioration. The P1 latency significantly prolonged with compression at all three levels
These N1 peak enlargements accompanied diminution of the P2 component, which was gradually replaced by the slower and prolonged downstroke of the N1 component (Figure 2A, oblique arrows). For example, the P2 component decreased in area toward final values of 17± 37% (P<0.003) at ‘−1’, 11±28% (P<0.002) at ‘−2’, and 39±26% (P=0.001) at ‘−3’ compared to the initial values. Caudal to the compression site, the P1 peak showed no significant change in amplitude and area and, as expected, in latency throughout the whole experiment (Figures 2A and 3B).
SCEPs recorded rostrally to the compression site
Enhancement of the N1 peak rostral to the compression site was less consistent. With the stepwise increase of the compressive load, the N1 peak significantly increased in amplitude and area, before subsequent deterioration, reaching maximal values of 107±3% (P<0.05) and 105±2% (P<0.01) at ‘+1’, 105±2% (P<0.05) and 106±2% (P<0.01) at ‘+2’, compared to the precompression values (Figures 2B and 3B). At ‘+3’, the N1 peak showed a significant increase in area to 104±2% (P<0.05), but not in amplitude. Again, the onset of this transient enlargement at ‘+1’, ‘+2’, and ‘+3’ levels, although varied among animals, correlated significantly (r=0.93 for amplitude; P<0.001, r=0.82 for area; P<0.001) with that of the prolongation in P1 latency at ‘+3’. The N1 peaks at these levels subsequently decreased and eventually were abolished.
The P1 peak showed a different time course depending on the recording site (Figure 2B). The amplitude and area of the P1 peak increased toward the final values of 144±17 and 245±20% at ‘+1’ or, conversely, decreased toward 37±5 and 50±9% at ‘+3’. At ‘+2’, there were both increase and decrease of the P1 peak depending on the cats under study, averaging 62±7% in amplitude and 105±9% in area for the final recordings. The P1 latency slightly but significantly (P<0.001) prolonged with compression by 0.19±0.01 ms at ‘+1’, by 0.20±0.01 ms at ‘+2’, and by 0.22±0.01 ms at ‘+3’, compared to the baseline values (Figure 3B).
Discussion
Spinal cord potentials recorded after epidurally applied cord stimulation in the midline normally consist of two major negative components. The first negative peak is mediated primarily by the dorsal spinocerebellar tract, while the second one is mediated by dorsal columns.14 In the present study, we placed both the stimulating and recording electrodes in the lateral epidural space to achieve a selective recording of the first component, thereby simplifying the waveform analysis. Under these experimental conditions, progressive cord compression paradoxically enhanced the N1 peaks of ascending SCEPs both caudally and, though less consistently, rostrally.
In previous animal studies, the enhancement of SCEPs was observed immediately caudal to the site of acute cord sectioning15 or subacute cord compression.12 A transient increase of SCEPs was also observed at the compression site.12 Recently, we reported results of the clinical study that described the paradoxical enhancement of negative peaks immediately caudal to the site of the conduction block.7 The present experimental study extends these observations and demonstrates caudal extension of the N1 peak enhancement at least down to the ‘−3’ level and rostral extension up to the ‘+3’ level. An enlargement of negative peak rostral to the compression has not been described previously.
What would be the mechanism according to which spinal cord compression causes the enhancement of N1 peaks? It is likely different from one responsible for the increase of the action current in demyelinated fibers at the lesion site as reported after chronic cord compression,16 because we dealt with subacute cord compression and detected enhancement both caudally and rostrally to the lesion site. Formation of the focal edema and change in physical characteristics of the volume conductor resulting from it is also unlikely to explain the nature of the enlargement confined to the negative peak at −3 through −1 and at +3. In fact, the onset of the N1 peak enhancement, whether recorded caudally or rostrally to the compression site, showed a close temporal correlation with that of the delay in P1 latency at the most rostral recording site. This prolongation in P1 latency could result from the loss of fast conducting fibers.17,18 Therefore, this finding suggests that conduction block or delay of fast conducting fibers across the compression site could play a role in producing the enhancement of the N1 peak. A computer model of a progressive conduction block supports this finding as well as predicts a similar phenomenon after the preferential block of fast fibers.10,11
How, then, do conduction abnormalities produce an enhancement of N1 peaks? It is known that SCEPs represent a linear summation of the nerve fiber action potentials (NFAPs) arising from constituent nerve fibers of different diameters. Normally, triphasic NFAPs with initial-positive, negative, and terminal-positive peaks are being produced at the time when the impulse approaches, then reaches, and finally passes beyond the point of recording (Figure 4).19 According to the concept of phase cancellation,20 which dictates the size of SCEPs, the overlap of NFAP peaks with opposite polarity results in physiologic cancellation and reduction in the negative peak of SCEPs (Figure 5a).
Square-wave solid-angle approximation predicting the triphasic potential generation in a volume conductor by a nerve impulse travelling from left to right. As shown in (a) and (b), the initial-positive peak is produced when the impulse approaches the recording electrode (G1). In (c), the negative peak is produced at the moment when the impulse reaches directly under G1. In (e) and (f), the terminal-positive peak is produce as the impulse passes beyond G1 (adapted from Kimura19)
A model for the phase interaction between fast and slow conducting fiber potentials as a mechanism of enhanced N1 peak of SCEPs because of conduction block or conduction delay in fast fibers. As shown in (a), the overlap of the NFAP peaks with opposite polarity normally results in cancellation, reducing the N1 peak of the SCEPs. In (b), as is the case with the recording caudal to compression, a conduction block of the fast fiber selectively removes its terminal-positive peak, resulting in N1 peak enhancement because of loss of phase cancellation. In (c), as is the case with the recording rostral to the compression site, a conduction block of the fast fiber removes not only its negative peak but also the terminal-positive peak, resulting in N1 peak enhancement because of loss of phase cancellation. In (d), a slight conduction delay of the fast fiber allows better synchronization of fast and slow fiber potentials, thereby enhancing the N1 peak
Immediately caudal to the compression site, the impulse in a blocked fiber reaches the recording site and gives rise to normal negativity, but does not move further away, thus failing to produce terminal positivity. This reduction of positive phases from blocked fibers can enhance the negativity of SCEPs resulting from the loss of physiologic phase cancellation (Figure 5b). Such a mechanism would account for the progressive enhancement of the N1 peak that was preserved even when every single fiber was blocked. Diminution of the P2 component (Figure 2A, oblique arrows) accompanied by N1 peak enhancement is also consistent with this view.
Rostral to the compression site, the impulse in a blocked fiber approaches without reaching the recording site. Impulses arriving at the site of conduction block will give rise to a killed-end effect with a local volume-conducted initial-positive wave alone10,11,21,22,23 (Figure 4b), which will quickly diminish in size rostrally; that is, removing both the negative and positive peaks. In this case, the loss of the negative peaks from blocked fibers would reduce the N1 peak. The loss of positive peaks from blocked fibers, however, can enhance the N1 peak. These two opposing factors would counter each other, tending to maintain the size of SCEPs despite the presence of conduction block.24 Thus, the transient N1 peak enhancement can be the result of a shift in the balance between two factors in favor of the negative potential of SCEPs (Figure 5c). This mechanism would account for the less consistent occurrence of N1 peak enhancement rostral to compression compared to caudal recordings, where the unopposed action of the loss of terminal-positive peaks from blocked fibers consistently enhances the N1 peak. Alternatively, a selective conduction delay of fast fibers can also lead to the enhancement of SCEPs resulting from better synchronization of the fast and slow fiber potentials (Figure 5d). This tendency to enhance the N1 peak, despite conduction abnormalities of fast fibers, breaks down with subsequent involvement of a greater number of nerve fibers, eventually causing decline toward abolition.
The P1 peak progressively diminished at ‘+3’, whereas it showed a progressive increase at ‘+1’. Similarly, an explanation for the P1 peak enhancement at ‘+1’ can be found using the concept of phase cancellation. As mentioned above, a blocked fiber gives rise to a volume-conducted initial-positive wave alone at ‘+1’ (Figure 4b), and only a very small positive wave at ‘+3’. At ‘+1’, unlike at ‘+3’, the absence of a negative wave accompanied by the nearly normal initial-positive wave from blocked fibers not only decreases the N1 peak of SCEPs, but also increases the P1 peak resulting from the loss of physiologic phase cancellation.
In conclusion, the findings presented here indicate that, in contrast to the popular belief, progressive cord compression can cause an enhancement of SCEPs. This phenomenon can be explained by the complex pattern of interaction between constituent nerve fibers of different diameters; that is, reduction in one polarity of constituent NFAPs may enhance the other polarity of SCEPs. This mechanism can cause the progressive increase of the N1 peak caudally and, though less consistently, the transient increase rostrally. An awareness of this possibility should be helpful in proper interpretation of electrophysiological data in intraoperative spinal cord monitoring and in accurate determination of the level of spinal cord lesion. In particular, this phenomenon can serve as a warning signal of the impending risk of paraplegia.
References
Waxman SG, Kocsis JD, Black JA . Pathophsiology of demyelinated axons. In: Waxman SG, Kocsis JD, Stys PK (eds). The Axons. Oxford University Press: New York 1995, pp 438–461.
Tamaki T et al. The prevention of iatrogenic spinal cord injury utilizing the evoked spinal cord potential. Int Orthop 1981; 4: 313–317.
Jones SJ et al. A system for the electrophysiological monitoring of the spinal cord during operations for scoliosis. J Bone Joint Surg (Br) 1983; 65: 134–139.
Satomi K et al. Level diagnosis of cervical myelopathy using evoked spinal cord potentials. Spine 1988; 13: 1217–1224.
Matsuda H, Shimazu A . Intraoperative spinal cord monitoring using electric responses to stimulation of caudal spinal cord or motor cortex. In: Desmedt JE (ed). Neuromonitoring in Surgery. Elsevier: New York 1989, pp 175–190.
Ohmi Y et al. Level diagnosis using spinal cord evoked potentials in cervical myelopath. In: Shimoji K, Kurokawa T, Willis Jr WD (eds). Spinal Cord Moni-toring and Electrodiagnosis. Springer-Verlag: Berlin 1991, pp 454–460.
Tani T et al. Waveform analysis of spinal somatosensory evoked potential: paradoxically enhanced negative peaks immediately caudal to the site of conduction block. Electroencephalogr Clin Neurophysiol 1998; 108: 325–330.
Tani T, Yamamoto H, Kimura J . Cervical spondylotic myelopathy in elderly people: a high incidence of conduction block at C3–4 or C4–5. J Neurol Neurosurg Psychiatry 1999; 66: 456–464.
Tani T et al. Intraoperative electroneurography in the assessment of the level of operation for cervical spondylotic myelopathy in the elderly. J Bone Joint Surg (Br) 2000; 82: 269–274.
Ushida T, Tani T . The spinal cord evoked potential by computer simulation: elucidation of killed-end potentials and augmentation caused by the conduction block phenomenon (in Japanese). Nippon Seikeigeka Gakkai Zasshi 1994; 68: 207–220.
Tani T et al. Waveform changes due to condution block and their underlying mechanism in spinal somatosensory evoked potential: a computer simulation. Technical note. J Neurosurg 1997; 86: 303–310.
Lee CS . The change of the spinal evoked potential caused by constant-speed subacute compression of the spinal cord (in Japanese). Nippon Seikeigeka Gakkai Zasshi 1985; 59: 949–960.
Shimoji K, Higashi H, Kano T . Epidural recording of spinal electrogram in man. Electroencephalogr Clin Neurophysiol 1971; 30: 236–239.
Tsuyama N et al. Clinical application os spinal cord action potential measurement. Int Orthop 1978; 2: 39–46.
Feldman MH et al. Spinal evoked potential in the monkey. Ann Neurol 1980; 7: 238–244.
Hoshino Y et al. Abnormally large evoked potentials arising from dorsal column fibers in the region of chronically compressed spinal cord. Clin Neurophysiol 1999; 110: 305–316.
Cummins KL, Perkel DH, Dorfman LJ . Nerve fiber conduction-velocity distributions. I. Estimation based on the single-fiber and compound action potentials. Electroencephalogr Clin Neurophysiol 1979; 46: 634–646.
Dorfman LJ . The distribution of conduction velocities (DCV) in peripheral nerves: a review. Muscle Nerve 1984; 7: 2–11.
Kimura J . Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. 3rd edn. Oxford University Press: New York 2001, pp 27–38.
Kimura J et al. Effect of desynchronized inputs on compound sensory and muscle actin potentials. Muscle Nerve 1988; 11: 694–702.
Woodbury JW . Potentials in a volume conductor. In: Ruch TC, Patton HD, Woodbury JW, Towe AL (eds). Neurophysiology 2nd edn. WB Saunders: Philadelphia 1965, pp 85–91.
Deecke L, Tator CH . Neurophysiological assessment of afferent and efferent conduction in the injured spinal cord of monekys. J Neurosurg 1973; 39: 65–74.
Schramm J, Shigeno T, Brock M . Clinical signs and evoked response alterations associated with chronic experimental cord compression. J Neurosurg 1983; 58: 734–741.
Tani T, Ushida T, Kimura J . Sequential changes of orthodromic sensory nerve action potentials induced by experimental compression of the median nerve at the wrist. Clin Neurophysiol 2001; 112: 136–144.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kishimoto, H., Tani, T., Ueta, E. et al. Paradoxical enhancement of spinal-cord-evoked potentials rostral and caudal to the site of progressive cord compression in the cat. Spinal Cord 41, 231–238 (2003). https://doi.org/10.1038/sj.sc.3101428
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101428