Abstract
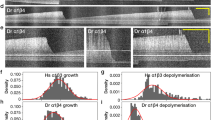
MICROTUBULES are involved in many important biological processes, including cell motility, cell division, morphogenesis and axonal transport, and it is of fundamental interest to understand the mechanism and regulation of tubulin polymerisation in order to clarify their function. Previously we presented evidence that brain tubulin, devoid of microtubule associated proteins (MAPs) after fractionation by phosphocellulose chromatography, exhibits a characteristic GTPase activity which is polymerisation-dependent and can be attributed to tubulin itself1. A detailed analysis of the steady-state GTPase activity led us to propose a model, according to which the rate of the polymerisation-dependent GTP hydrolysis should be proportional to the product of the concentration of microtubule ends and of tubulin-GTP. At the polymerisation equilibrium, the concentration of tubulin dimers is just equal to the ‘critical concentration’2, which is the minimum concentration above which tubulin polymers exist. Therefore, at the polymerisation equilibrium, the rate of GTP hydrolysis should be directly proportional to the concentration of microtubule ends. We now present evidence consistent with this prediction, from experiments in which mictrotubules were fragmented by sonication to increase the number of microtubule ends and the resulting change in the rate of GTP hydrolysis was measured.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
David-Pfeuty, T., Erickson, H. P. & Pantaloni, D. Proc. natn. Acad. Sci. U S.A. 74, 5372–5375 (1977).
Gaskin, F., Cantor, C. R. & Shelanski, M. L. J. molec. Biol. 89, 737–758 (1974).
Shelanski, M. L., Gaskin, F. & Cantor, C. R. Proc. natn. Acad. Sci. U.S.A. 70, 765–768 (1973).
Murphy, D. B. & Borisy, G. G. Proc. natn. Acad. Sci. U.S.A. 72, 2696–2700 (1975).
Weingarten, M. D., Lockwood, A. H., Hwo, S. & Kirschner, M. W. Proc. natn. Acad. Sci. U.S.A. 72, 1858–1862 (1975).
Erickson, H. P. & Voter, W. A. Proc. natn. Acad. Sci. U.S.A. 73, 2813–2817 (1976).
Lee, J. C. & Timasheff, S. N. Biochemistry 14, 5183–5187 (1975).
Fellows, A., Francon, J., Lennon, A. M. & Nunez, J. Eur. J. Biochem. 78, 167–174 (1977).
Murphy, D. B., Johnson, K. A. & Borisy, G. G. J. Cell Biol. 70, 225a (1976).
Oosawa, F. J. theor. Biol. 27, 69–86 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DAVID-PFEUTY, T., LAPORTE, J. & PANTALONI, D. GTPase activity at ends of microtubules. Nature 272, 282–284 (1978). https://doi.org/10.1038/272282a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/272282a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.