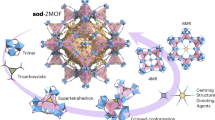
Abstract
The increasing demand for enantiomerically pure chemicals has stimulated extensive research into the preparation of heterogeneous chiral catalysts or separation media that combine both shape selectivity and enantioselectivity1. Helical pores in inorganic materials might be able to perform such functions, but their occurrence is rare1. Attempts have been reported to synthesize a specific enantiomorph of the chiral zeolite beta2, and chiral metal complexes have been used to assemble inorganic precursors into chiral frameworks3,4. Materials with a fully three-dimensional array of helical structural units are particularly rare, because helical structures (such as quartz) are commonly generated by a uni-dimensional symmetry element acting on an achiral structural subunit5,6. Here we report on a family of zeolite-type materials (which we call UCSB-7) that possess two independent sets of three-dimensional crosslinked helical pores, separated by a gyroid periodic minimal surface13. We have synthesized the UCSB-7 framework for various compositions (zinc and beryllium arsenates, gallium germanate) using either inorganic cations or amines as structure-directing agents. The helical-ribbon motif that we identify might be exploited more widely for developing useful chiral solid-state structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Baiker, A. Chiral catalysis on solids. Curr. Opin. Solid State Mater. Sci. 3, 86–93 (1998).
Davis, M. E. New vistas in zeolite and molecular sieve catalysis. Acc. Chem. Res. 26, 111–115 (1993).
Bruce, D. A., Wilkinson, A. P., White, M. G. & Bertrand, J. A. The synthesis and characterization of an aluminophosphate with chiral layers: trans-Co(dien)2·Al3P4O16·3H2O. J. Solid State Chem. 125, 228–233 (1996).
Morgan, K., Gainsofrd, G. & Milestone, N. Anovel layered aluminum phosphate [Co(en) Al3P4O16·3H2O] assembled about a chiral metal complex. J. Chem. Soc. Chem. Commun. 425–426 (1995).
Harrison, W. T. A., Gier, T. E., Stucky, G. D., Broach, R. W. & Bedard, R. A. NaZnPO4·H2O, an open-framework sodium zincophosphate with a new chiral tetrahedral framework topology. Chem. Mater. 8, 145–151 (1996).
Soghomonian, V., Chen, Q., Haushalter, R. C., Zubieta, J. & O'Connor, C. J. An inorganic double helix:hydrothermal synthesis, structure, and magnetism of chiral [(CH3)2NH2]K4[V10O10(H2O)2(OH)4(PO4)7]·4H2O. Science 259, 1596–1599 (1993).
Bu, X., Feng, P., Gier, T. E. & Stucky, G. D. Two ethylenediamine templated zeolite type structure sin zinc arsenate and cobalt phosphate systems. J. Solid State Chem. 136, 210–215 (1998).
Smith, J. V. Topochemistry of zeolites and related materials. 1. Topology and geometry. Chem. Rev. 88, 148–182 (1988).
Meier, W. M., Olson, D. H. & Baerlocher, C. Atlas of Zeolite Structure Types (Elsevier, Boston, (1996)).
Bu, X., Feng, P. & Stucky, G. D. Large-cage zeolite structures with multidimensional 12-ring channels. Science 278, 2080–2085 (1997).
Monnier, A.et al. Cooperative formation of inorganic–organic interfaces in the synthesis of silicate mesostructures. Science 261, 1299–1303 (1994).
Andersson, S. & Jacob, M. The Mathematics of Structures (R. Oldenbourg, München, (1997)).
Andersson, S., Hyde, S. T., Larsson, K. & Lidin, S. Minimal surfaces and structures: from inorganic and metal crystals to cell membranes and biopolymers. Chem. Rev. 88, 221–242 (1988).
Nenoff, T. M.et al. Structural and chemical investigations of Na3(ABO4)3·4H2O-type sodalite phases. Inorg. Chem. 33, 2472–2480 (1994).
Harrison, W. T. A., Gier, T. E. & Stucky, G. D. Two lithium chloroberyllo(phosphate/arsenate) sodalites: Li4Cl(BePO4)3and Li4Cl(BeAsO4)3. Acta Crystallogr. C 50, 471–473 (1994).
Feng, P., Bu, X. & Stucky, G. D. Hydrothermal synthesis and structural characterization of zeolite analogue compounds based on cobalt phosphate. Nature 388, 735–741 (1997).
Acknowledgements
This research was supported in part by the National Science Foundation and the Office of Naval Research. P.F. thanks the Materials Research Laboratory for a Corning Foundation Fellowship. All figures were generated using software programs from Molecular Simulations.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Rights and permissions
About this article
Cite this article
Gier, T., Bu, X., Feng, P. et al. Synthesis and organization of zeolite-like materials with three-dimensional helical pores. Nature 395, 154–157 (1998). https://doi.org/10.1038/25960
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/25960
This article is cited by
-
Design and synthesis of polyoxometalate-framework materials from cluster precursors
Nature Reviews Materials (2017)
-
Enhanced H2S gas-sensing properties of Pt-functionalized In2Ge2O7 nanowires
Applied Physics A (2014)
-
A Flexible Hexacarboxylate-Samarium(III) Metal–Organic Framework: Synthesis, Structure and Spectroscopic Properties
Journal of Inorganic and Organometallic Polymers and Materials (2013)
-
Roles of trifluoroacetic acid, acetic acid and their salts in the synthesis of helical mesoporous materials
Journal of Porous Materials (2010)
-
Self-assembly of bridged silsesquioxanes incorporated with conjugated organic functionalities
Frontiers of Chemistry in China (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.