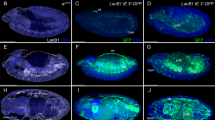
Abstract
The homeobox gene caudal (cad) has a maternal embryonic function that establishes the antero–posterior body axis of Drosophila1,2. It also has a conserved2,4 late embryonic and imaginal function1 related to the development of the posterior body region. Here we report the developmental role of cad in adult Drosophila. It is required for the normal development of the analia structures, which derive from the most posterior body segment. In the absence of cad function, the analia develop like the immediately anterior segment (male genitalia), following the transformation rule of the canonical Hox genes5. We also show that cad can induce ectopic analia development if expressed in the head or wing. We propose that cad is the Hox gene that determines the development of the fly's most posterior segment. cad acts in combination with the Hedgehog (Hh) pathway6 to specify the different components of the analia: the activities of cad and of the Hh pathway induce Distal-less expression that, together with cad, promote external analia development. In the absence of the Hh pathway, cad induces internal analia development, probably by activating the brachyenteron and even-skipped genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Macdonald, P. & Struhl, B. Amolecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature 324, 537–545 (1986).
Mlodzik, M., Fjose, A. & Gehring, W. J. Isolation of caudal, a Drosophila homeobox-containing gene with maternal expression, whose transcription forms a concentration gradient at the pre-blastoderm stage. EMBO J. 4, 2961–2969 (1985).
Hunter, C. P. & Kenyon, C. Spatial and temporal controls target pal-1 blastomere-specification activity to a single blastomere lineage in C. elegans embryos. Cell 87, 217–226 (1997).
Brooke, N. M., Garcia-Fernandez, J. & Holland, P. W. The ParaHox gene cluster is an evolutionary sister of the Hox gene cluster. Nature 392, 920–922 (1998).
McGinnis, W. & Krumlauf, R. Homeobox genes and axial patterning. Cell 68, 283–302 (1992).
Basler, K. & Struhl, G. Compartment boundaries and the control of Drosophila limb pattern by Hedgehog protein. Nature 368, 208–214 (1994).
Wu, L. H. & Lengyel, J. A. Role of caudal in the hindgut specification and gastrulation suggests homology between Drosophila amnioproctodeal invagination and vertebrate blastopore. Development 125, 2433–2442 (1996).
Calleja, M., Moreno, E., Pelaz, S. & Morata, G. Visualization of gene expression in living adult Drosophila. Science 274, 252–255 (1996).
Casares, F., Sanchez, L., Guerrero, I. & Sanchez-Herrero, E. The genital disc of Drosophila melanogaster. I. Segmental and compartmental organization. Dev. Genes Evol. 207, 216–228 (1997).
Nöthiger, R., Dübendorfer, A. & Epper, F. Gynandromorphs reveal two separate primordia for male and female genitalia in Drosophila melanogaster. Wilhelm Roux Arch. Dev. Biol. 181, 367–373 (1977).
Epper, F. Three-dimensional fate map of the female genital disc of Drosophila melanogaster. Wilhelm Roux Arch. Dev. Biol. 192, 270–274 (1983).
Cohen, S. M., Broner, G., Kuntter, F., Jurgens, G. & Jackle, H. Distal-less encodes a homeodomain protein required for limb development in Drosophila. Nature 338, 432–434 (1989).
Frasch, M., Hoey, T., Rushlow, C., Doyle, H. & Levines, M. Characterization localization of the even-skipped protein of Drosophila. EMBO J. 6, 749–759 (1987).
Kispert, A., Herrmann, B. G., Leptin, M. & Reuter, R. Homologs of the mouse Brachyury gene are involved in the specification of posterior terminal structures in Drosophila, Tribolium, and Locusta. Genes Dev. 8, 2137–2150 (1994).
Singer, J. B., Harbecke, R., Kusch, T., Reuter, R. & Lengyel, J. A. Drosophila brachyenteron regulates gene activity and morphogenesis in the gut. Development 122, 3703–3718 (1996).
Nusslein-Volhard, C., Kluding, H. & Jurgens, G. Genes affecting the segmental subdivisions of the Drosophila embryo. Cold Spring Harb. Symp. Quant. Biol. 50, 145–154 (1985).
Sanchez-Herrero, E., Vernos, I., Marco, R. & Morata, G. Genetic organization of the Drosophila bithorax complex. Nature 313, 108–113 (1985).
Lecuit, T. & Cohen, S. M. Proximal–distal axis formation in the Drosophila leg. Nature 388, 139–145 (1997).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Diaz-Benjumea, F. J., Cohen, B. & Cohen, S. M. Cell interaction between compartments establishes the proximal–distal axis of Drosophila legs. Nature 372, 175–179 (1994).
Alexandre, C., Jacinto, A. & Ingham, P. W. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the Cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 10, 2003–2013 (1996).
van den Heuvel, M. & Ingham, P. W. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature 382, 547–551 (1996).
Chawengsaksophak, K., James, R., Hammond, V. E., Kontgen, F. & Beck, F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 396, 84–87 (1997).
Miller, D. J. & Miles, A. Homeobox genes and the zootype. Nature 365, 215–216 (1993).
Sánchez, L. Casares, F., Gorfinkiel, N. & Guerrero, I. The genital disc of Drosophila melanogaster. II. Role of the genes hedgehog, decapentaplegic and wingless. Dev. Genes Evol. 207, 229–241 (1997).
Murakami, R. et al. aproctous, a locus that is necessary for the development of the proctodeum in Drosophila embryos, encodes a homolog of the vertebrate Brachyury gene. Wilhelm Roux Arch. Dev. Biol. 205, 89–96 (1995).
Gorfinkiel, N., Morata, G. & Guerrero, I. The homeobox gene Distal-less induces ventral appendage development in Drosophila. Genes Dev. 11, 2259–2271 (1997).
Diaz-Benjumea, F. & Cohen, S. M. Serrate signals through Notch to establish a wingless-dependent organizer at the dorsal-ventral compartment boundary of the Drosophila wing. Development 121, 4215–4225 (1995).
Acknowledgements
We thank P. Fernandez, T. Kusch, C. Parras, R. Rivera, I. Rodriguez and G. Struhl for antibodies, cDNAs and fly stocks; J. Casanova and E. Sanchez-Herrero for comments on the manuscript; S. Gonzalez for helping with the DNA injections; and R. Gonzalez and J. M. Galán for their technical help. This work was supported by grants from the Dirección General de Investigación Cientifica y Técnica and from the Human Frontier Science Program. An institutional grant from the Fundacion Ramon Areces to the Centro de Biologia Molecular is also acknowledged. E.M. is supported by a scholarship from the Comunidad Autónoma de Madrid.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreno, E., Morata, G. Caudal is the Hox gene that specifies the most posterior Drosophile segment. Nature 400, 873–877 (1999). https://doi.org/10.1038/23709
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/23709
This article is cited by
-
Sex determining systems of Drosophila control somatic sexual differentiation by cytoplasmic determinants and inductive signals: an insight from pattern development of aberrant sexual genotypes
The Nucleus (2023)
-
Comparative genomics of the coconut crab and other decapod crustaceans: exploring the molecular basis of terrestrial adaptation
BMC Genomics (2021)
-
Mutation of amphioxus Pdx and Cdx demonstrates conserved roles for ParaHox genes in gut, anus and tail patterning
BMC Biology (2020)
-
Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals
Nature Neuroscience (2013)
-
Usefulness of endoscopic brushing and magnified endoscopy with narrow band imaging (ME-NBI) to detect intestinal phenotype in columnar-lined esophagus
Journal of Gastroenterology (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.