Abstract
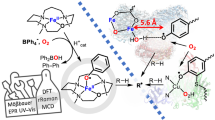
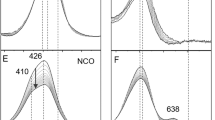
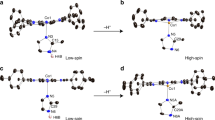
THE reversible reaction of certain cobalt chelates with molecular oxygen can be considered as a simple model of the reversible oxygen binding by haemoglobin and myoglobin1,2. It was recently pointed out3–6 that in suitable experimental conditions synthetic oxygen carriers show a reaction stoichiometry corresponding to one oxygen molecule per metal atom. In both cases the six-coordinated complex with oxygen can be formed by an equatorial tetradentate chelating agent and a donor ligand in the axial position trans to oxygen4. The reversibility of oxygenation seems to depend on a non-polar environment of the metal provided by the aprotic solvents in the simple complexes and by the hydrophobic pocket accommodating the haem in haemoglobin7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bayer, E., and Schretzmann, P., Structure and Bonding, 2, 181 (1967).
Bigotto, A., Costa, G., Mestroni, G., Pellizer, G., Puxeddu, A., Reisenhofer, E., Stefani, L., and Tauzher, G. (in the press).
Floriani, C., and Calderazzo, F., J. Chem. Soc., A, 946 (1969).
Costa, G., Puxeddu, A., and Nardin-Stefani, L., Inorg. Nucl. Chem. Lett., 6, 191 (1970).
Crumbliss, A. L., and Basolo, F., J. Amer. Chem. Soc., 92, 55 (1970).
Green, M., and Mettrick, D., Inorg. Nucl. Chem. Lett., 6, 149 (1970).
Perutz, M. F., Muirhead, M., Cox, J. M., and Goaman, L. C. G., Nature, 219, 131 (1968).
Morgan, G., and Smith, J., J. Chem. Soc., 2030 (1925).
Rossi-Fanelli, A., and Antonini, E., Arch. Biochem. Biophys., 80, 229 (1958).
Hughes, E. W., Wilmarth, W. K., and Calvin, M., J. Amer. Chem. Soc., 68, 2273 (1946).
Harle, O. L., and Calvin, M., J. Amer. Chem. Soc., 68, 2612 (1946).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
AMICONI, G., BRUNORI, M., ANTONINI, E. et al. Thermodynamics of the Reversible Oxygenation of Bis (Acetylacetone)-Ethylenediiminecobalt (II) in Pyridine. Nature 228, 549–551 (1970). https://doi.org/10.1038/228549a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/228549a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.