Abstract
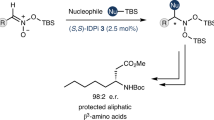
THE o-nitrophenylsulphenyl (NPS) residue has recently been introduced as an amine-protecting group for amino-acids and peptides during peptide synthesis by Zervas et al.1. It is readily cleaved by mild acidolysis using 2–3 equivalents of hydrochloric acid in ether. Although this treatment allows the selective cleavage of the o-nitrophenylsulphenamide group in the presence of acid-labile tert-butylesters in the peptide moiety under carefully controlled conditions2 another cleavage procedure avoiding the use of acid altogether might prove useful.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Zervas, L., Borovas, D., and Gazis, E., J. Amer. Chem. Soc., 85, 3660 (1963).
Zervas, L., Photaki, I., Poduska, K., and Sheppard, B. C., Proc. Seventh European Peptide Symp., Budapest, 1964, Acta Chim. Acad. Sci. Hung. (in the press).
For reviews compare Pettit, G. R., and Van Tamelen, E. E., in Org. Reactions, 12, 356 (1962). Hauptmann, H., and Walther, W. F., Chem. Rev., 62, 347 (1962).
McKay, F. C., and Albertson, N. F., J. Amer. Chem. Soc., 79, 4686 (1957); Anderson, G. W., and McGregor, A. C., 79, 6180 (1957).
Roeske, R., Chem. and Indust., 1121 (1959). Anderson, G. W., and Callahan, F. M., J. Amer. Chem. Soc., 82, 3359 (1960).
Beyerman, H. C., and Bontekoe, J. S., Proc. Chem. Soc., 249 (1961); Rec. Trav. Chim. Pays-Bas, 81, 691, 699 (1962). Schröder, E., Liebigs Ann. Chem., 670, 127 (1963). Callahan, F. M., Anderson, G. W., Paul, R., and Zimmerman, J. A., J. Amer. Chem. Soc., 85, 201 (1963).
Dane, E., Drees, F., Konrad, P., and Dockner, T., Angew. Chem., 74, 873 (1962).
Anderson, G. W., Zimmerman, J. E., and Callahan, F. M., J. Amer. Chem. Soc., 85, 3039 (1963); 86, 1839 (1964).
Anderer, F. A., et al., Nature, 186, 922 (1960); Z. Naturforschg., 17, b, 536 (1962). Tsugita, A., et al., Proc. U. S. Nat. Acad. Sci., 46, 1463 (1960).
Prepared according to Billica, H. R., and Adkins, H., Organic Syntheses Coll., 3, 176 (1955).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MEIENHOFER, J. Cleavage of o-Nitrophenylsulphenamides by Raney Nickel and Applications for Peptide Synthesis. Nature 205, 73–75 (1965). https://doi.org/10.1038/205073b0
Published:
Issue Date:
DOI: https://doi.org/10.1038/205073b0
This article is cited by
-
Synthesis of dinitrophenyltetrapeptides as chromophoric substrates of endoproteinases
Chemistry of Natural Compounds (1983)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.