Abstract
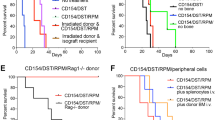
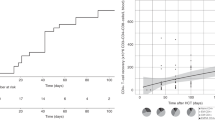
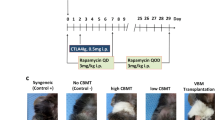
We compared the effects of intraosseous BMT with those of standard i.v. BMT on the efficacy on donor-cell engraftment into the BM and lymphoid organs across an MHC barrier in rats. Twenty-four intraosseous and 24 i.v. BMTs were performed from 48 ACI (RT1a) donors to 48 Lewis (RT1l) recipients. Each transplant group received either intraosseous or i.v. BMT. Groups I and II served as controls without immunosuppression (n=16); groups III and IV received cyclosporine monotherapy (n=16); and V and VI received αβ-TCR monoclonal antibody and cyclosporine A (αβ-TCR/CsA) for 7 days (n=16). In each group, four rats received 35 × 106 transplanted bone marrow cells (BMCs) and four received 70 × 106 cells. All animals survived without GVHD. Mean (±s.d.) donor-cell engraftment into BM of recipients after intraosseous BMT was 7.9% (±1.3%) in recipients receiving αβ-TCR-CsA and 70 × 106 BMCs, and 4.2% (±1.4%) in recipients after i.v. transplantation. The seeding efficacy of donor cells into lymphoid tissue was greater after intraosseous BMT and αβ-TCR-CsA than after standard i.v. transplantation. In our model, intraosseous BMT facilitated donor-cell engraftment under short-term immunodepletive αβ-TCR/CsA protocol, which resulted in a temporary state of immune unresponsiveness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ricordi C, Karatzas T, Nery J, Webb M, Selvaggi G, Fernandez L et al. High-dose donor bone marrow infusions to enhance allograft survival: the effect of timing. Transplantation 1997; 63: 7–11.
Ciancio G, Miller J, Garcia-Morales RO, Carreno M, Burke III GW, Roth D et al. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation 2001; 71: 827–835.
Garcia-Morales R, Carreno M, Mathew J, Zucker K, Cirocco R, Ciancio G et al. Continuing observations on the regulatory effects of donor-specific bone marrow cell infusions and chimerism in kidney transplant recipients. Transplantation 1998; 65: 956–965.
van der Loo JC, Ploemacher RE . Marrow- and spleen-seeding efficiencies of all murine hematopoietic stem cell subsets are decreased by preincubation with hematopoietic growth factors. Blood 1995; 85: 2598–2606.
Cashman JD, Eaves CJ . High marrow seeding efficiency of human lymphomyeloid repopulating cells in irradiated NOD/SCID mice. Blood 2000; 96: 3979–3981.
Castello S, Podesta M, Menditto VG, Ibatici A, Pitto A, Figari O et al. Intra-bone marrow injection of bone marrow and cord blood cells: an alternative way of transplantation associated with a higher seeding efficiency. Exp Hematol 2004; 32: 782–787.
Nakamura K, Inaba M, Sugiura K, Yoshimura T, Kwon AH, Kamiyama Y et al. Enhancement of allogenic hematopoietic stem cell engraftment and prevention of GVHD by intra-bone marrow bone marrow transplantation plus donor lymphocyte infusion. Stem Cell 2004; 22: 125–134.
Askenasy N . Localized bone marrow transplantation leads to skin allograft acceptance in nonmyeloablated recipients: comparison of intra-bone marrow and isolated limb perfusion. Stem Cell 2002; 20: 86–93.
Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y et al. SCID-repopulating cell activity of human cord blood-derived CD34-cells assured by intra-bone marrow injection. Blood 2003; 101: 2924–2931.
Yahata T, Ando K, Sato T, Miyatake H, Nakamura Y, Muguruma Y et al. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood 2003; 101: 2905–2913.
Esumi T, Inaba M, Ichioka N, Kushida T, Iida H, Ikehara S . Successful allogenic leg transplantation in rats in conjunction with intra-bone marrow injection of donor bone marrow cells. Transplantation 2003; 76: 1543–1548.
Ikehara S . A novel strategy for allogenic stem cell transplantation: perfusion method plus intra-bone marrow injection of stem cells. Exp Hematol 2003; 31: 1142–1146.
Mahmud N, Pang W, Cobbs C, Alur P, Borneman J, Dodds R et al. Studies of the route of administration and role of conditioning with radiation on unrelated allogenic mismatched mesenchymal stem cell engraftment in a nonhuman primate model. Exp Hematol 2004; 32: 494–501.
Siemionow MZ, Klimczak A, Unal S . Different routes of donor-derived hematopoietic stem cell transplantation for donor-specific chimerism induction across MHC barrier. Transplant Proc 2005; 37: 62–64.
Ramsamooj R, Llull R, Black KS, Hewitt CW . Composite tissue allografts in rats: IV. Graft-versus-host disease in recipients of vascularized bone marrow transplants. Plast Reconstr Surg 1999; 104: 1365–1371.
Beschorner WE, Tutschka PJ, Santos GW . Chronic graft-versus-host disease in the rat radiation chimera. I. Clinical features, hematology, histology, and immunopathology in long-term chimeras. Transplantation 1982; 33: 393–399.
Hardy CL, Tavassoli M . Homing of hematopoietic stem cells to hemopoietic stroma. Adv Exp Med Biol 1988; 241: 129–133.
van Hennik PB, de Koning AE, Ploemacher RE . Seeding efficiency of primitive human hematopoietic cells in nonobese diabetic/severe combined immune deficiency mice: implications for stem cells frequency assessment. Blood 1999; 94: 3055–3061.
Hashimoto F, Sigiura K, Inoue K, Ikehara S . Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vivo. Blood 1997; 89: 49–54.
Vos O, Buurman WA, Ploemacher RE . Mobilization of haemopoietic stem cells (CFU) into the peripheral blood of the mouse: effects of endotoxin and other compounds. Cell Tissue Kinet 1972; 5: 467–479.
Siemionow M, Ortak T, Izycki D, Oke R, Cunningham B, Prajapati R et al. Induction of tolerance in composite-tissue allografts. Transplantation 2002; 74: 1211–1217.
Siemionow MZ, Izycki DM, Zielinski M . Donor-specific tolerance in fully major histocompatibility complex-mismatched limb allograft transplants under an anti-αβ T-cell receptor monoclonal antibody and cyclosporine A protocol. Transplantation 2003; 76: 1662–1668.
Huang Y, Cramer DE, Ray MB, Chilton PM, Que X, Ildstad ST . The role of alphabeta- and gammadelta-T cells in allogenic donor marrow on engraftment, chimerism, and graft-versus-host disease. Transplantation 2001; 72: 1907–1914.
Herve P . Donor-derived hematopoietic stem cells in organ transplantation: technical aspects and hurdles yet to be cleared. Transplantation 2003; 75 (9 Suppl): 55S–57S.
Murase N, Starzl TE, Tanabe M, Fujisaki S, Miyazawa H, Ye Q et al. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to brown Norway rats. Transplantation 1995; 60: 158–171.
Jiang Z, Adams GB, Hanash AM, Scadden DT, Levy RB . The contribution of cytotoxic and noncytotoxic function by donor T-cells that support engraftment after allogeneic bone marrow transplantation. Biol Blood Marrow Transplant 2002; 8: 588–596.
Siemionow MZ, Ozer K, Izycki D . A new method of bone marrow transplantation leads to extension of skin allograft survival. Transplant Proc 2005; 37: 2309–2314.
Fan TX, Hisha H, Jin TN, Yu CZ, Lian ZX, Guo SB et al. Successful allogeneic bone marrow transplantation (BMT) by injection of bone marrow cells via portal vein: stromal cells as BMT-facilitating cells. Stem Cell 2001; 19: 144–150.
Talmor M, Steinman RM, Codner MA, Chen M, Harper AD, Witmer-Pack MD et al. Bone marrow-derived chimerism in non-irradiated, cyclosporine-treated rats receiving microvascularized limb transplants: evidence for donor-derived dendritic cells in recipient lymphoid tissues. Immunology 1995; 86: 448–455.
Hagglund H, Ringden O, Agren B, Wennberg L, Remberger M, Rundquist L et al. Intraosseous compared intravenous infusion of allogenic bone marrow. Bone Marrow Transplant 1998; 21: 331–335.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klimczak, A., Unal, S., Jankowska, A. et al. Donor–origin cell engraftment after intraosseous or intravenous bone marrow transplantation in a rat model. Bone Marrow Transplant 40, 373–380 (2007). https://doi.org/10.1038/sj.bmt.1705743
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705743
Keywords
This article is cited by
-
The Positive Impact of Donor Bone Marrow Cells Transplantation into Immunoprivileged Compartments on the Survival of Vascularized Skin Allografts
Archivum Immunologiae et Therapiae Experimentalis (2021)
-
Donor Recipient Chimeric Cells Induce Chimerism and Extend Survival of Vascularized Composite Allografts
Archivum Immunologiae et Therapiae Experimentalis (2021)
-
Immunomodulatory Effects of Different Cellular Therapies of Bone Marrow Origin on Chimerism Induction and Maintenance Across MHC Barriers in a Face Allotransplantation Model
Archivum Immunologiae et Therapiae Experimentalis (2016)
-
Twenty-year follow-up of a randomized trial comparing intraosseous and i.v. BM transplantation
Bone Marrow Transplantation (2014)