Summary:
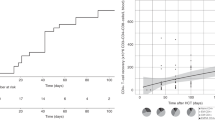
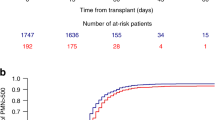
Growth factors are routinely used after autotransplantation to accelerate hematopoietic recovery, and are continued until the absolute neutrophil count (ANC) is ⩾0.5 × 109/l on 3 consecutive days. Since ANC often increases to very high levels with this strategy, we discontinued growth factor on the first day ANC reached 0.5 × 109/l in 45 patients (Study Group), and compared their subsequent ANC to 108 historic controls who received growth factor longer. While ANC on the day after reaching 0.5 × 109/l was comparable between groups, ANC on the third day was significantly higher in the Control Group (2.3 vs 4.9 × 109/l; P=0.0003). When compared to the first day, ANC in the Study Group was higher by a median of 140% on the third day and by 450% in the Control Group (P=0.0002). A significantly higher proportion of patients experienced a decline in ANC after the first day in the Study Group. However, only one patient in the Study Group became neutropenic transiently and ANC recovered spontaneously the next day. The incidence of fever and hospitalization were comparable. We conclude that growth factors can be discontinued after autotransplantation the day the ANC reaches 0.5 × 109/l, without compromising neutrophil recovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Schmitz N, Dreger P, Zander AR et al. Results of a randomised, controlled, multicentre study of recombinant human granulocyte colony-stimulating factor (filgrastim) in patients with Hodgkin's disease and non-Hodgkin's lymphoma undergoing autologous bone marrow transplantation. Bone Marrow Transplant 1995; 15: 261–266.
Gorin NC, Coiffier B, Hayat M et al. Recombinant human granulocyte-macrophage colony-stimulating factor after high-dose chemotherapy and autologous bone marrow transplantation with unpurged and purged marrow in non-Hodgkin's lymphoma: a double-blind placebo-controlled trial. Blood 1992; 80: 1149–1157.
Torres-Gomez A, Jimenez MA, Alvarez MA et al. Optimal timing of granulocyte colony-stimulating factor (G-CSF) administration after bone marrow transplantation. A prospective randomized study. Ann Hematol 1995; 71: 65–70.
Faucher C, Le Corroller AG, Chabannon C et al. Administration of G-CSF can be delayed after transplantation of autologous G-CSF-primed blood stem cells: a randomized study. Bone Marrow Transplant 1996; 17: 533–536.
Stahel RA, Jost LM, Honegger H et al. Randomized trial showing equivalent efficacy of filgrastim 5 micrograms/kg/d and 10 micrograms/kg/d following high-dose chemotherapy and autologous bone marrow transplantation in high-risk lymphomas. J Clin Oncol 1997; 15: 1730–1735.
Bolwell B, Goormastic M, Dannley R et al. G-CSF post-autologous progenitor cell transplantation: a randomized study of 5, 10, and 16 micrograms/kg/day. Bone Marrow Transplant 1997; 19: 215–219.
Bolwell BJ, Pohlman B, Andresen S et al. Delayed G-CSF after autologous progenitor cell transplantation: a prospective randomized trial. Bone Marrow Transplant 1998; 21: 369–373.
Ali MY, Oyama Y, Monreal J et al. Reassessing the definition of myeloid engraftment after autotransplantation: it is not necessary to see 0.5 × 109/L neutrophils on 3 consecutive days to define myeloid recovery. Bone Marrow Transplant 2002; 30: 749–752.
Rihn C, Cilley F, Monreal J et al. Definition of myeloid engraftment after allogeneic hematopoietic stem cell transplantation. Blood 2002; 100: 417a–418a, (abstract #1618).
Ali MY, Oyama Y, Monreal J et al. Ideal or actual body weight to calculate CD34+ cell doses for autologous hematopoietic stem cell transplantation? Bone Marrow Transplant 2003; 31: 861–864.
Spitzer TR . Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27: 893–898.
San Miguel JF, Hernandez MD, Gonzalez M et al. A randomized study comparing the effect of GM-CSF and G-CSF on immune reconstitution after autologous bone marrow transplantation. Br J Haematol 1996; 94: 140–147.
Acknowledgements
This work was supported in part by the Auxiliary Board of the Northwestern Memorial Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, A., Pedicano, J., Trifilio, S. et al. How long after neutrophil recovery should myeloid growth factors be continued in autologous hematopoietic stem cell transplant recipients?. Bone Marrow Transplant 33, 715–719 (2004). https://doi.org/10.1038/sj.bmt.1704415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704415
Keywords
This article is cited by
-
Actual or ideal body weight to calculate CD34+ cell dose in patients undergoing autologous hematopoietic SCT for myeloma?
Bone Marrow Transplantation (2009)
-
G-CSF or not G-CSF? That is the question
Bone Marrow Transplantation (2004)