Summary:
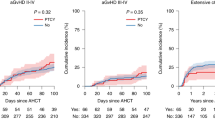
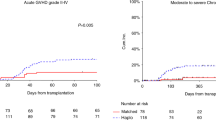
A total of 31 consecutive patients with hematologic malignancies who were considered poor candidates for TBI underwent allogeneic stem cell transplantation after conditioning with fludarabine and melphalan. A total of 25 matched sibling recipients received fludarabine 25 mg/m2 × 5 days and melphalan 70 mg/m2 × 2 days. For unrelated and haploidentical donor recipients, fludarabine was increased to 30 mg/m2 and ATG 30 mg/kg × 4 days was added. Graft-versus-host disease prophylaxis consisted of tacrolimus and mini methotrexate. All patients engrafted. Regimen-related toxicity was considerable and included mainly renal, hepatic and mucosal toxicity. There were seven regimen-related-deaths including two VOD, two pulmonary, one renal, one cardiac and one mucosal toxicity. One case of fatal pulmonary toxicity death could be attributed to pre-existing pulmonary damage. Progression-free survival at 12 months was 44% (90% CI: 30–58%) for recipients of HLA-identical sibling transplants and 33% (90% CI: 21–45%) for all patients. In conclusion, the fludarabine–melphalan regimen leads to consistent engraftment. The regimen-related toxicity is considerable and cannot be explained solely by patient selection. Cardiac toxicity is emerging as a unique toxicity of this regimen. Despite toxicity, fludarabine–melphalan has considerable activity and leads to durable remission in a proportion of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Giralt S, Thall PF, Khouri I et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001; 97: 631–637.
Slavin S, Nagler A, Naparstek E et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
McSweeney PA, Niederwieser D, Shizuru JA et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Devine SM, Sanborn R, Jessop E et al. Fludarabine and melphalan-based conditioning for patients with advanced hematological malignancies relapsing after a previous hematopoietic stem cell transplant. Bone Marrow Transplant 2001; 28: 557–562.
Przepiorka D, Khouri I, Ippoliti C et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after HLA-mismatched marrow or blood stem cell transplantation. Bone Marrow Transplant 1999; 24: 763–768.
Volpi I, Perruccio K, Tosti A et al. Postgrafting administration of granulocyte colony-stimulating factor impairs functional immune recovery in recipients of human leukocyte antigen haplotype-mismatched hematopoietic transplants. Blood 2001; 97: 2514–2521.
Przepiorka D, Ippoliti C, Panina A et al. Ganciclovir three times per week is not adequate to prevent cytomegalovirus reactivation after T-cell depleted marrow transplantation. Bone Marrow Transplant 1994; 13: 461–464.
Bearman SI, Appelbaum FR, Buckner CD et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
Przepiorka D, Weisdorf D, Martin P et al. Consensus conference on GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Papadopoulos EB, Carabasi MH, Castro-Malaspina H et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood 1998; 91: 1083–1090.
Soiffer RJ, Fairclough D, Robertson M et al. CD6-depleted allogeneic bone marrow transplantation for acute leukemia in first complete remission. Blood 1997; 89: 3039–3047.
Hansen JA, Gooley TA, Martin PJ et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med 1998; 338: 962–968.
Thomas ED . Bone marrow transplantation for malignant disease. J Clin Oncol 1983; 1: 517.
Bensinger WI, Martin PJ, Storer B et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 2001; 344: 175–181.
Champlin RE, Schmitz N, Horowitz MM et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT). Blood 2000; 95: 3702–3709.
Samuels BL, Britan JD . High-dose intravenous melphalan: a review. J Clin Oncol 1995; 13: 1786–1799.
Van Besien KW, Demuynck H, LeMaistre CF et al. High dose melphalan allows durable engraftment of allogeneic bone marrow. Bone Marrow Transplant 1995; 15: 321–323.
Giralt S, Estey E, Van Besien KW et al. Induction of graft-versus-leukemia without myeloablative therapy using allogeneic pbsc after purine analog containing regimens. Blood 1996; 88(Suppl. 1): 614a (Abstr.).
van Besien K, Devine S, Wickrema A et al. Fludarabine Melphalan is a suitable alternative to TBI-based conditioning in patients with advanced hematologic malignancies. Proceedings of 7th Annual Meeting of the European Hematology Association, 2002, pp. 135–142.
Sarosy G, Leyland-Jones B, Soochan P, Cheson BD . The systemic administration of intravenous melphalan. J Clin Oncol 1988; 6: 1768.
Ho VT, Soiffer RJ . The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 2001; 98: 3192–3204.
Chao N . Pharmacology and use of immunosuppressive agents after hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ (eds). Hematopoietic Cell Trans-plantation. Blackwell Sciences, Inc.: Malden, MA, 1999, pp 176–185.
Morgenstern GR, Powles R, Robinson B, Mc Elwain TJ . Cyclosporin interaction with ketoconazole and melphalan. Lancet 1982; 2: 1342.
Ritchie DS, Seymour JF, Roberts AW et al. Acute left ventricular failure following melphalan and fludarabine conditioning. Bone Marrow Transplant 2001; 28: 101–103.
Martino R, Caballero MD, Canals C et al. Allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning: results of a prospective multicentre study. Br J Haematol 2001; 115: 653–659.
Ferrara JLM, Antin JH . The pathophysiology of graft vs host disease. In: Thomas ED, Blume KG, Forman SJ (eds). Hematopoietic Cell Transplantation. Blackwell Sciences: Malden, MA, 1999, pp 305–315.
Przepiorka D, Saliba R, Anderlini P et al. Chronic graft vs host disease after allogeneic stem cell transplantation. Blood 2001; 98: 1695–1700.
Folz RJ . Mechanisms of lung injury after bone marrow transplantation. Am J Respir Cell Mol Biol 1999; 20: 1097–1099.
Kottaridis PD, Milligan DW, Chopra R et al. In vivo CAMPATH-1 H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000; 96: 2419–2425.
Robinson SP, Mackinnon S, Goldstone A et al. Higher than expected transplant-related mortality and relapse following non-myeloablative stem cell transplantation for lymphoma adversely affects survival. Blood 2000; 96(Suppl. 1): (Abstr. 2380). p. 554a.
Yunis JJ, Brunning RD, Howe RB, Lobell M . High-resolution chromosomes as an independent prognostic indicator in adult acute nonlymphocytic leukemia. N Engl J Med 1984; 311: 812.
Bacigalupo A . Second EBMT Workshop on reduced intensity allogeneic hemopoietic stem cell transplants (RI-HSCT). Bone Marrow Transplant 2002; 29: 191–195.
Author information
Authors and Affiliations
Additional information
Supported in part by a grant from Berlex Pharmaceuticals
Rights and permissions
About this article
Cite this article
van Besien, K., Devine, S., Wickrema, A. et al. Regimen-related toxicity after fludarabine–melphalan conditioning: a prospective study of 31 patients with hematologic malignancies. Bone Marrow Transplant 32, 471–476 (2003). https://doi.org/10.1038/sj.bmt.1704166
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704166
Keywords
This article is cited by
-
Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation
Journal of Thrombosis and Thrombolysis (2021)
-
Cardiac Complications in the Adult Bone Marrow Transplant Patient
Current Oncology Reports (2019)
-
Fludarabine and neurotoxicity in engineered T-cell therapy
Gene Therapy (2018)
-
Cardiovascular Complications of Hematopoietic Stem Cell Transplantation
Current Treatment Options in Cardiovascular Medicine (2016)
-
Comparison of reduced conditionings combining fludarabine with melphalan or 3-day busulfan in patients allografted for myeloid neoplasms
International Journal of Hematology (2014)