Abstract
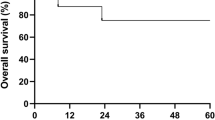
A female patient with AML received an allogeneic BMT from her brother. She experienced two relapses managed with chemotherapy and donor leukocyte infusions. The patient subsequently developed extensive therapy-refractory chronic GVHD. Pseudoautologous blood stem cell transplantation was performed as a salvage treatment for chronic GVHD. Her blood stem cells were easily mobilized with cyclophosphamide and G-CSF. The conditioning regimen was well tolerated and consisted of 200 mg/kg cyclophosphamide and horse-derived antithymocyte globulin. A total of 4.03 × 106/kg CD34+ cells were infused and hematological recovery was rapid. Chronic GVHD improved with the ability to taper steroids. Nine months post transplantation the patient died from leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tyndall A, Fassas A, Passweg J et al. Autologous hematopoietic stem cell transplants for autoimmune disease-feasibility and transplant-related mortality Bone Marrow Transplant 1999 24: 729 734
McSweeney PA, Furst DE, Nash RA et al. High-dose immunosuppressive therapy (HDIT) and autologous stem cell transplantation for severe systemic sclerosis (SSc) Blood 2000 96: 844a
Burt RK, Traynor AE, Pope R et al. Treatment of autoimmune disease by intense immunosuppressive conditioning and autologous hematopoietic stem cell transplantation Blood 1998 92: 3505 3514
Binks M, Passweg JR, Furst D et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease Ann Rheum 2001 60: 577 584
Ochs LA, Blazar BR, Roy J et al. Cytokine expression in human cutaneous chronic graft-versus-host disease Bone Marrow Transplant 1996 17: 1085 1092
Pavletic S, O'Dell J, Pirruccello S et al. Intensive immunoablation and autologous blood stem cell transplantation in patients with refractory rheumatoid arthritis: The University of Nebraska Experience J Rheumatol 2001 28: (Suppl. 64) 13 20
Parkman R . Chronic graft-versus-host disease Curr Opin Hematol 1998 5: 22 25
Brodsky RA, Petri M, Smith BD et al. Immunoablative high-dose cyclophosphamide without stem-cell rescue for refractory, severe autoimmune disease Ann Intern Med 1998 129: 1031 1035
Baron F, Gothot A, Salmon J-P et al. Clinical course and predictive factors for cyclosporine-induced autologous graft-versus-host disease after autologous hematopoietic stem cell transplantation Br J Haematol 2000 111: 745 753
Guillaume T, Rubinstein DB, Symann M . Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation Blood 1998 92: 1471 1490
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pusic, I., Pavletic, S., Kessinger, A. et al. Pseudoautologous blood stem cell transplantation for refractory chronic graft-versus-host disease. Bone Marrow Transplant 29, 709–710 (2002). https://doi.org/10.1038/sj.bmt.1703550
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703550
Keywords
This article is cited by
-
Successful pseudo-autologous stem cell transplantation for donor-derived Burkitt lymphoma occurring 9 years after allogeneic transplantation
International Journal of Hematology (2023)
-
Cyclophosphamide for salvage therapy of chronic graft-versus-host disease: a retrospective analysis
Annals of Hematology (2020)
-
Pseudo-autologous stem cell transplantation for donor-derived mantle cell lymphoma 12 years after allogeneic transplantation
International Journal of Hematology (2018)
-
Pulse cyclophosphamide for corticosteroid-refractory graft-versus-host disease
Bone Marrow Transplantation (2005)
-
Reversal of severe graft-versus-host disease after nonmyeloablative matched unrelated donor stem cell transplant by infusion of backup autologous peripheral blood stem cells
Bone Marrow Transplantation (2005)