Abstract
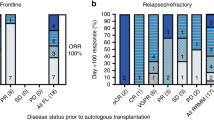
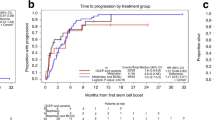
This study was designed to determine the maximum tolerated dose (MTD) of high-dose melphalan (HDM), with peripheral blood stem cell support, that could be given twice within 90 days to patients with multiple myeloma. Twenty patients received tandem HDM at 160, 180 or 200 mg/m2 and a total of 55 were treated at the estimated MTD of 200 mg/m2. Seventeen of 55 (31%) did not receive cycle 2; six because of low CD34+ cell yields, three because of severe (n = 1) or fatal toxicities (n = 2) and eight for other reasons. The median interval between doses for 38 patients was 70 days (range 41–225). Three of 55 patients (5%) died of treatment-related causes. In patients completing two cycles of HDM, at any dose level, the complete remission rate improved from 15% following cycle 1 to 55% following cycle 2. The probabilities of survival, event-free survival and relapse or progression at 18 months for the 55 patients treated at the MTD were 0.84, 0.76 and 0.20, respectively, with a median follow-up of 19 months (range 9–36) from mobilization chemotherapy. It was concluded that two cycles of HDM, 200 mg/m2, could be administered to approximately 70% of patients under the age of 66 with multiple myeloma in a median interval of 70 days, with improvement in CR rates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weaver, C., Zhen, B., Schwartzberg, L. et al. Phase I–II evaluation of rapid sequence tandem high-dose melphalan with peripheral blood stem cell support in patients with multiple myeloma. Bone Marrow Transplant 22, 245–251 (1998). https://doi.org/10.1038/sj.bmt.1701324
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1701324
Keywords
This article is cited by
-
A new conditioning regimen involving total marrow irradiation, busulfan and cyclophosphamide followed by autologous PBSCT in patients with advanced multiple myeloma
Bone Marrow Transplantation (2003)
-
Dexamethasone, paclitaxel, etoposide, cyclophosphamide (d-TEC) and G-CSF for stem cell mobilisation in multiple myeloma
Bone Marrow Transplantation (2001)
-
Mobilization of peripheral blood stem cells following myelosuppressive chemotherapy: a randomized comparison of filgrastim, sargramostim, or sequential sargramostim and filgrastim
Bone Marrow Transplantation (2001)
-
Prolonged survival after intensive therapy and purged ABMT in patients with multiple myeloma
Bone Marrow Transplantation (2000)