Abstract
Objective:
The purpose of this investigation was to verify if avoidance of allergenic foods in children adhering to a food allergen avoidance diet from birth was complete and feasible, and whether dietary assessment can be used as a tool in predicting the outcome of double-blind, placebo-controlled food challenges (DBPCFCs).
Design:
Children adhering to an allergen avoidance diet from birth underwent DBPCFCs. The investigator-dietician verified whether the elimination was complete, using food frequency questionnaires for common allergenic foods.
Setting:
University Medical Centre Groningen, the Netherlands.
Subjects:
Thiry-eight children aged 1–13 years, who were consecutively referred to the University Medical Centre Groningen for DBPCFC between January 2002 and February 2004.
Results:
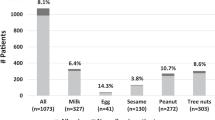
Among the 38 children undergoing DBPCFCs, there were 15 challenges with egg, 15 with peanut, five with hazelnut and three with soy. Fifteen food challenges (39%) were positive. Small quantities of allergenic foods were inadvertently present in the diets of 13 patients (34%), were possibly present in the diets of 14 patients (37%) and could not be identified in the diets of 11 patients (29%). Seven patients (54%) who had inadvertently ingested small quantities of allergenic foods without sequelae had a positive DBPCFC.
Conclusion:
Dietary avoidance was incomplete and not feasible in most cases. Tolerance of small amounts of allergenic foods does not preclude positive challenge reactions. Dietary assessment does not seem a useful tool in predicting the outcome of DBPCFC in children adhering to an elimination diet.
Sponsorship:
The Stichting Astma Bestrijding (Foundation for the Prevention of Asthma), The Netherlands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Altshul AS, Scherrer DL, Muñoz-Furlong A, Sicherer SH (2001). Manufacturing and labelling issues for commercial products: relevance to food allergy. J Allergy Clin Immunol 108, 468.
American Academy of Pediatrics, Committee on Nutrition (2000). Hypoallergenic infant formulas. Pediatrics 106, 346–349.
Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J et al. (2004). Standardization of food challenges in patients with immediate reactions to foods – position paper from the European Academy of Allergology and Clinical Immunology. Allergy 59, 690–697.
Bruynzeel-Koomen C, Ortolani C, Aas K, Bindslev-Jensen C, Bjorksten B, Moneret-Vautrin D et al. (1995). Adverse reactions to food. European Academy of Allergology and Clinical Immunology Subcommittee. Allergy 50, 623–635.
Commissie ‘Standaard’ (2005). Landelijke standaard voedselallergie bij zuigelingen. Standaard voor de diagnose, behandeling en preventie van voedselallergie bij zuigelingen op het consultatiebureau [Committee on food hypersensitivity. Nation-wide standard for the diagnosis, treatment and prevention of food hypersensitivity in infants at the well-baby clinic]. 5th ed. Den Haag: Voedingscentrum.
European Parliament and Council (2003). Directive 2003/89/EC amending Directive 2000/13/EC. Official Journal of the European Union L 308, 25.11.03, p. 15.
Fukushima Y, Kawata Y, Onda T, Kitagawa M (1997). Consumption of cow milk and egg by lactating women and the presence of β-lactoglobulin in breast milk. Am J Clin Nutr 65, 30–35.
Gowland MH (2001). Food allergen avoidance – the patient's viewpoint. Allergy 56 (Suppl 67), 117–120.
Høst A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P et al. (1999). Dietary products used in infants for treatment and prevention of food allergy. Joint statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on hypoallergenic formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on nutrition. Arch Dis Child 81, 80–84.
Joshi P, Modifi S, Sicherer SH (2002). Interpretation of commercial food ingredient labels by parents of food-allergic children. J Allergy Clin Immunol 109, 1019–1021.
Lack G, Fox D, Northstone K, Golding J (2003). Factors associated with the development of peanut allergy in childhood. N Engl J Med 348 (11), 977–985.
Muraro A, Dreborg S, Halken S, Høst A, Niggeman B, Aalberse R et al. (2004). Dietary prevention of allergic diseases in infants and small children. Part III: Critical review of published peer-reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol 15, 291–307.
Sampson HA (2001). Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 107, 891–896.
Taylor SL, Hefle SL (2001). Ingredient and labelling issues associated with allergenic foods. Allergy 56 (Suppl 67), 64–69.
Vierk K, Falci K, Wolyniak C, Klontz KC (2002). Recalls of foods containing undeclared allergens reported to the US Food and Drug Administration, fiscal year 1999. J Allergy Clin Immunol 109, 1022–1026.
Vlieg-Boerstra BJ, Bijleveld CMA, van der Heide S, Beusekamp BJ, Wolt-Plompen SAA, Kukler J et al. (2004). Development and validation of challenge materials for double-blind, placebo-controlled food challenges in children. J Allergy Clin Immunol 113, 341–346.
World Allergy Organization, Johansson SGO, Haahtela T (eds). (2004). World Allergy Organization guidelines for prevention of allergy and allergic asthma Condensed version. Int Arch Allergy Immunol 135, 83–92.
Wood RA (2002). Food manufacturing and the allergenic consumer: Accidents waiting to happen. J Allergy Clin Immunol 109, 920–922.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: BJ Vlieg-Boerstra
Contributors: BJV-B was responsible for the study design, the performance of the study and the writing of the paper, under direct supervision of AEJD and EJD. CMAB, Svan der H and SAAW-P collaborated in the study design, discussion and interpretation of the results and comments upon the manuscript. JK performed the food challenges.
Rights and permissions
About this article
Cite this article
Vlieg-Boerstra, B., van der Heide, S., Bijleveld, C. et al. Dietary assessment in children adhering to a food allergen avoidance diet for allergy prevention. Eur J Clin Nutr 60, 1384–1390 (2006). https://doi.org/10.1038/sj.ejcn.1602468
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602468
Keywords
This article is cited by
-
Consultation with registered dietitian to prevent accidental reactions to food: insight from an egg allergy influenza vaccination cohort
European Journal of Clinical Nutrition (2017)
-
The development of a standardised diet history tool to support the diagnosis of food allergy
Clinical and Translational Allergy (2015)
-
Cow's milk allergy in children: adherence to a therapeutic elimination diet and reintroduction of milk into the diet
European Journal of Clinical Nutrition (2010)