Abstract
The aim of this study was to examine the potential of serial rCBF studies to directly characterize the regional effects and dynamic time course of the centrally active drug ketamine. The value of a broader application of this technique to other neurally active drugs to characterize the pharmacodynamics of CNS compounds is suggested by these data. Thirteen normal subjects received a 0.3 mg/kg intravenous dose of ketamine over 60 seconds; ten other individuals received placebo in the same manner. For each subject, three baseline PET rCBF scans and seven sequential post-ketamine scans at 10-minute intervals were obtained using H215O water. SPM techniques were employed to identify the maxima of any cluster significant by spatial extent analysis at any post-ketamine time point between 0 and 36 min. These extremes from the ketamine group, were identified in placebo scans similarly and grown to a 6x6x12 mm voxel set. The average rCBF values of the ketamine-defined clusters were determined in the drug and placebo conditions at all time points. rCBF across time was plotted for each cluster and compared between drug and placebo. Area under the curve (AUC) was calculated between baseline and 36 minutes. The kinetic characteristics of the ketamine-induced rCBF curves were compared to induced behaviors in each maxima. Ketamine produced distinct patterns of rCBF change over time in different brain regions; maxima within an anatomically defined region responded similarly. Ketamine induced rCBF activations in anterior cingulate, medial frontal and inferior frontal cortices. All maxima with a relative flow reduction with ketamine were in the cerebellum. The pattern of all activations and suppressions was monophasic with the peak changes at 6–16 minutes. In preliminary analysis, individual Cmax and AUC of maxima in the anterior cingulate/medial frontal region tended to correlate with the mild psychotomimetic action of ketamine; whereas, there was no tendency toward correlation with this psychological change in cerebellar maxima. The direct action of a centrally active drug can be assessed regionally and dynamically in brain using rCBF and a scan sequence optimally timed to complement the drug's time course. Ketamine pharmacodynamic response can be related to concurrent behavioral changes, tending to link the behavior with a brain region. This experimental design provides direct characterization of drug action in the CNS in ways heretofore unavailable.
Similar content being viewed by others
Main
The actions of most psychotropic drugs on the central nervous system (CNS) are regionally selective. Many drugs target neurotransmitter receptors or synaptic reuptake sites as their sites of action, and these are regionally distributed in unique patterns in brain (Goodman-Gilman 1995). Thus, the functional actions of centrally active drugs are likely to show characteristic regional distributions on indices of neuronal activity in CNS. The CNS location of functional drug action has already been examined for several different drug families; these functional patterns are pharmacologically distinctive and behaviorally predictive in laboratory animals and in humans (Tamminga et al. 1987; Weissman et al. 1987; Buchsbaum et al. 1987; Cavazzuti et al. 1987; Lahti et al. 1995a; Holcomb et al. 1996). The dynamic time course of such drug-induced physiological changes could add an additional parameter of interest to the localization information. They indicate the course as well as the region of the drug action, clarifying the drug's physiological disposition with respect to time.
Pharmacokinetic studies of drug plasma levels have previously provided the primary kinetic information used to understand the dynamics of drug action in brain (VanPutten et al. 1991). However, direct dynamic measures of tissue response at the cerebral site of action could supplement plasma kinetic analyses, as an informative pharmacodynamic characterization of a compound. rCBF measurements provide a potentially useful technique to directly assess regional and functional CNS tissue response to a neurally active drug pharmacodynamically (Friston et al. 1994a). Temporally sequential rCBF acquisitions have the particular advantage of providing time sensitive and anatomically specific pharmacodynamic data.
Drug action in the brain is localized and time-dependent. Occupancy of a receptor by a drug or selective ligand will initially determine the anatomic distribution and time course of that drug-receptor effect. But, the functional changes induced by a drug will not be restricted to the primary receptor's distribution or the duration of the receptor-ligand interaction, because of the influence of cerebral systems on overall drug response. Nonetheless, drug induced change patterns, indicated by rCBF fluctuations, can be regionally specific and have distinctive regional time characteristics, especially informative where the action is at variance with the simple receptor profile.
Ketamine [(2-chlorphenyl-2-methylamino-cyclohexanone)] is an anesthetic agent, commonly used in pediatric surgery and occasionally for obstetrical or dental procedures (White et al. 1982). It has a redistribution half life of 11 minutes and an elimination half life of 2.5 hours. Also, the drug produces a selective, mild and time-limited stimulation of psychotic symptoms in schizophrenic individuals (Lahti et al. 1995b) and mild psychotomimetic effects in normal adults (Krystal et al. 1994; Malhotra et al. 1996; Breier et al. 1997). We and others have attributed these effects to ketamine's antagonist action at the central NMDA-sensitive glutamate receptor because of the occurrence of these mental phenomena at relatively low doses (Tamminga 1998).
The time course of behavioral response to ketamine is 15–30 minutes, with cessation of the behavioral expression of drug effect by 20–35 minutes after administration (Lahti et al. 1995b). We have reported that after an acute bolus administration of ketamine, the drug produces elevated rCBF in the anterior cingulate and right frontal cortex, and reduced rCBF in the cerebellum, lingual gyrus, and hippocampus (Lahti et al. 1995b).
This report presents the analysis of acute ketamine action on rCBF changes over time in a cohort of normal healthy subjects. Regional blood flow was assessed using H215O2-water at 10-minute intervals for 36 minutes following drug administration. Baseline flow rates were contrasted with the serial post-drug measurements following injection of active drug or placebo.
METHODS
Subjects and Assessments
Twenty-three healthy volunteers were recruited from the community through newspaper advertizing. Thirteen received ketamine (0.3 mg/kg) and 10 received saline, each blind to drug condition. Age, sex distribution, and highest level of education achieved were similar in both groups: Ketamine group, age = 31.3 ± 7.5 years old; five males, eight females; 17.1 ± 3.0 years of education; Placebo group, age = 29.9 ± 6.7 years old; five males, five females; 15.4 ± 1.8 years of education, respectively. Each subject was screened for medical and psychiatric illness by telephone, and interviewed in person using the SCID to rule out any primary psychiatric diagnosis; all volunteers with a family history of major psychiatric illness in a first degree relative were excluded.
Each volunteer agreed to participate in this study after being fully informed about risks and potential benefits. Each volunteer received ketamine (0.3 mg/kg) and placebo under research conditions in our laboratory with psychological evaluation, prior to the PET evaluation reported here, to reduce any novelty response. The psychological effects of ketamine were completely reviewed in the consent process and described in detail in the written consent form to ensure the full informing of volunteers. The protocol was reviewed, approved, and followed by the University of Maryland Human Volunteers Committee and the Johns Hopkins Joint Committee on Clinical Investigation. All subjects denied previous ketamine use, either illicit or prescribed, when specifically asked.
Scan Technique
A GE 4096+ PET camera was used in conjunction with the bolus water method, 62.5 mCi per tracer injection. Volunteers were positioned in the camera using a thermoplastic face mask. The most inferior plane was placed at 20 mm above the canthomeatal axis. After subjects were comfortably positioned in the scanner three baseline scans were completed, 10 minutes apart. Scan data were acquired for 60 seconds, beginning 20 sec after tracer infusion. Seven minutes prior to the fourth scan ketamine (0.3 mg/kg) was infused into an antecubital vein over 60 seconds. Consequently, the first post-ketamine scan was acquired 6 minutes after completing the ketamine administration. Subsequent scans were acquired 16, 26, and 36 minutes post-drug. Magnetic resonance images were acquired on a GE Signa magnetom, using spoiled gradient, 3-dimensional T1 weighted parameters. These structured images were used for single subject analyses.
Image Analysis
Image data were transferred to a SUN Sparc 10 work station where SPM96 software was used to assess changes in rCBF between the baseline scans and subsequent post-ketamine studies.
Analyses were performed in three steps. First, positive and negative contrasts were made between the three baseline scans (averaged) and each of the four post-ketamine scans, after which the initial activation/suppression returned to baseline. RCBF in the three baseline scans were not significantly different from each other when tested pixel by pixel, thus they were averaged. These contrasts used alpha levels of p < .05 (corrected for multiple comparisons), and z-score thresholds of z = 3.09. Significance was assessed by spatial extent methodology (Friston et al. 1994b). The maximum change voxel location x, y, z coordinate in Talairach Atlas space (Talairach and Tournoux 1988) was identified within each significant cluster at each of the four time points following ketamine infusion (6, 16, 26, and 36 minutes post drug infusion). Significant maxima were first identified in scan 4 (6 min). At subsequent scan times, distinct maxima were deemed different from the 6 min maxima, if located more than one full width half maxima (FWHM) away from a previously identified point. The three most significant maxima from every significant cluster were selected for analysis.
Second, a 3 × 3 × 3 voxel box (6 × 6 × 2 mm) was used to represent and average the space around each maxima in the significant clusters found at the four post-ketamine time points. The average rCBF of the voxels in each sampling box was determined at the post-drug time points and at baseline. For the placebo analyses, the maxima derived in the ketamine analyses were applied to the control condition to generate the control values at each time point for each of the ketamine-induced maxima. These points were analyzed with a 2 (ketamine, saline) by 7 (scans) repeated measures analysis of variance (α = 0.05). Ketamine-induced rCBF changes were manifest during the first 36 min after drug administration and returned to baseline thereafter. Therefore, differences in rCBF between ketamine and placebo over time (6–36 min) were addressed for each region. Significant time by drug interactions were explored with Bonferroni corrected post-hoc comparisons.
Third, the pharmacodynamic characteristics of these “grown maxima” were assessed kinetically for each individual and averaged for each group. The WinNonlin program (Pharsight Corp., Mountain View, CA) was used to identify the peak (Tmax and Cmax), and area under the curve (AUC) for the rCBF-time plots. Absolute magnitude of rCBF changes was used and any single value in an opposite direction was changed to 0. Kinetic characteristics at each significant maxima were correlated with the psychosis subscale of the BPRS in each subject. Some subjects' data were excluded from these analyses because a pharmacodynamic model could not be fit to their rCBF data.
RESULTS
SPM Analysis of Ketamine Scans
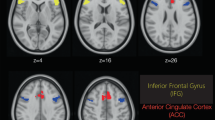
Analysis of the rCBF scans after placebo demonstrated no significant activation or inhibition clusters at any time point in the post-placebo scans. Figure 1 presents the SPM projection images of the ketamine-condition showing positive change maps taken at each of the activated post-drug time points (6–36 min) for the volunteers receiving ketamine. At each time point representative transverse plots illustrating significant areas of activation are superimposed on a representative MRI (Figure 2) . In the first post-ketamine scan at 6 minutes both the anterior cingulate and the right middle frontal cortex show enhanced flow. Ten minutes later, the anterior cingulate is still activated but in a smaller area. At 26 minutes post-drug, activation in the cingulate has diminished, but an area in left parietal cortex has activated. At 36 minutes no significant flow increases are apparent. The activation curves in anterior cingulate cortex, right middle frontal and left parietal areas, are monophasic.
Volumetric, “bird-cage” views of significant rCBF changes are presented. Four time points were sampled following ketamine administration. Activation clusters represent significant changes, positive on the left and negative on the right, at each time point contrasted against the baseline. Positive changes exhibit a monotonic decline with respect to time in the anterior cingulate and frontal regions. Negative changes similarly decline in the cerebellum. Reduced rCBF foci were also observed in the primary visual cortex
Ketamine induced positive, upper, and negative, lower panel, rCBF changes are graphically superimposed on representative magnetic resonance images of the brain. These demonstrate the strong initial activation in the anterior cingulate and frontal cortex, and the rCBF suppression in the cerebellum. Different levels were chosen to represent the maximum activation sites at each time point
Analyses of rCBF inhibition are also plotted in Figure 1. Representative transverse plots indicate the significant inhibition areas, overlaid on an MRI (Figure 2). At 6 minutes, blood flow is diminished throughout the cerebellum. The suppression continues into the 16-minutes scan. By 26 and 36 minutes the areas of flow reduction have become more restricted but are still significant. The maxima involved in this response describe a monophasic response. SPM images from control cases show no significant areas of flow diminution. rCBF patterns return to baseline values by the 36-minute scan.
rCBF in Ketamine-Induced Activation/Inhibition Clusters: Ketamine Versus Placebo
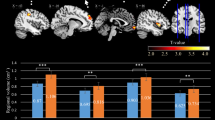
All distinct clusters where ketamine significantly activated or inhibited rCBF according to criteria over the 36 minutes of analysis were analyzed. Individual rCBF values of the clusters at each time point were identified, averaged, and plotted over time in the ketamine compared with the placebo groups. Several of these clusters failed to show a significant drug x time interaction: specifically these were cerebellum (Tailarach coordinates: −52, 2, −12), cerebellum (10, −72, −12), brainstem (−6, 0, −12), and the inferior frontal (58, 24, 12). All other clusters showed significant ketamine-placebo differences over time. The six activated clusters are located in medial frontal, anterior cingulate and inferior frontal cortex (Figure 3). The three inhibited clusters are located in cerebellum (Figure 4).
Positive changes in rCBF, 5 to 10 percent, are presented for specific locations in the cingulate and frontal cortex. The solid line represents the rCBF associated with the ketamine active condition, and the dotted line represents the activity at the specified site during the placebo condition. All foci exhibited significant changes
Negative changes in rCBF, 5 to 10 percent, are presented for three separate sites in the cerebellum of subjects who either received ketamine or placebo. Ketamine activations are graphed with a solid line and placebo is graphed with a dotted line. Only foci in the cerebellum were significantly different from placebo, with regard to the negative rCBF changes
Quantification of the rCBF Area Under the Curves
The dynamic blood flow versus time data were analyzed by noncompartmental pharmacokinetic analyses, using the nonlinear least-squares regression computer program, WinNonlin. The observed parameters included the maximum blood flow (Cmax) and time to maximum flow (Tmax). The area under the curve (AUC) was calculated by the linear trapezoidal rule between BL and 36 min (Table 1) . These analyses were done at each of the significant ketamine-determined maximum between BL and 36 min. The primary activation/inhibition peaks were manifest and reduced by 36 minutes.
Behavioral Response
The ketamine group evidenced a significant, albeit mild, increase in the BPRS score (mean = +10.6; range = +2 to +20; BPRS score) but the placebo group did not (mean = −0.9; range = −5 to +1; BPRS score). The change in the total BPRS score was wholly accounted for by an increase in the psychosis items. The kinetic characteristics of each individual curve from the ketamine group were correlated in a preliminary fashion with the psychosis subscale of the BPRS (BPRS-P). Despite having only 13 subjects to include in the analysis, trend correlations developed (Table 2) . In the anterior cingulate cortex cluster, two maxima showed a trend toward a positive linear correlation between the AUC or Cmax and the ketamine-induced behavior (3B cmax; r = 0.567, df = 12, p = .0413; 3B, AUC, r = 0.508, df = 12, p = .076). In cerebellum no trend emerged.
DISCUSSION
The data presented here with their different analyses show several distinctive aspects of the drug-induced rCBF changes over time. The sequential SPM analyses of each time point show qualitatively how the shape and significance of the areas of activation or suppression change over time. The cluster criteria for these SPM projection images are significant by the spatial extent method. This analysis provides a qualitative time-dependent presentation of ketamine-responsive brain areas.
Elaborated by quantitative kinetic analysis, the dynamic sequence of the maxima provides an overall description of location, extent, and duration of drug effect. The lack of any significant clusters in the placebo condition or any cluster growth and regression over time suggests that the activation/ reduction patterns depicted here are associated with ketamine drug action.
The “grown maxima” rCBF plotted over time, reflect the magnitude and dynamic course of ketamine action in drug-responsive brain regions. Multiple maxima within a single anatomically defined region in frontal cortex or cerebellum showed similar rCBF response with respect to magnitude and direction over time. There was variability between anatomic areas in the magnitude and direction of these functional responses. The responses within the anterior cingulate and cerebellar cortex suggest that regions within these areas are dynamically distinct.
The kinetic analysis, including the Cmax, Tmax, and the AUC of significant maxima, integrates and quantifies the time and magnitude changes between and across subjects. The kinetic analysis of drug action can demonstrate and quantify individual and group variability. These calculations, performed on an even larger group of individuals will help to describe the range of functional ketamine-induced changes in a normal human population. The trend correlations between rCBF in selected maxima in frontal cortex/anterior cingulate and behavior suggest that these measurements may help to link functions and regions. Because of this drug's effects on mental status (Krystal et al. 1994; Lahti et al. 1995b; Malhotra et al. 1996) ketamine may provide a probe to advance an understanding of psychosis from a dynamic physiological point of view. It has been particularly useful to determine to what extent a regional blood flow change pattern is associated with the subjective psychotic mental changes.
We have previously reported that volunteers with schizophrenia show the same large increase in anterior cingulate cortex demonstrated here with healthy volunteers (Lahti 1995b). The rCBF decrease previously reported in that study in hippocampus was not replicated here, likely due to the partial volume effect with this small structure. In another analysis, using ROI techniques, an MRI template, and individual placement, we have subsequently confirmed the observation that ketamine reduces rCBF in human hippocampus, but only in schizophrenic volunteers.
The time interval employed in a drug's rCBF evaluation needs to be determined relative to its behavioral and kinetic half life. In the case of ketamine, its half life is short and its central drug action begins within seconds after administration; the psychotomimetic action begins by 1–2 min and the duration of its behavioral effects extends 20–30 minutes after its infusion. By 35 minutes, the volunteer's mental status has returned to normal. The time course of the scan analyses spans the duration of CNS effects of the drug. The relatively short behavioral time course of ketamine makes it ideal for the time limited rCBF study shown here. A similar assessment of drugs with longer drug half-lives and pharmacologic actions will require a longer scan schedule.
Ketamine is a noncompetitive antagonist at the NMDA receptor complex and acts to block ion flow through the NMDA-gated ionophore. The distribution of the NMDA receptor is broad with highest NMDA densities in cerebral cortex including hippocampus and moderate density in subcortical regions (Whetsell and Shapira 1993; Greenamyre and Porter 1994; Monyer et al. 1994). However, the ketamine-induced rCBF activation patterns do not simply follow the cerebral distribution patterns of the NMDA receptor. rCBF change after ketamine is much more restricted (Lahti et al. 1995a; Breier et al. 1997).
If the ketamine-induced rCBF patterns fail to follow the NMDA receptor distribution, the question becomes what accounts for the rCBF pattern described by ketamine. Clearly, NMDA receptor composition and function varies regionally across cortical areas, an observation which could account for this phenomenon (Priestley and Kemp 1993; Burdett et al. 1995; Porter and Greenamyre 1995). Also, racemic ketamine was administered here, not the pure R or S isomer; hence, the rCBF response patterns reflect the actions of both isomers (Vollenweider et al. 1997). But, also the answer might lie not in the NMDA receptor-rich regions, but in the glutamate-responsive cerebral circuits. System circuitry within the limbic cortex may well determine a regional neuronal population response large enough to be registered as rCBF activation with this conservative SPM imaging analysis. Based on other informative studies, we have speculated that primary hippocampal actions of ketamine might induce the anterior cingulate rCBF activation (Tamminga 1998). Although the latter formulation is speculative, additional study can test this formulation. Moreover, the use of fMRI in future studies to sample drug-induced rCBF curves, will provide a more precise time curve hence a more precise tool to answer questions of drug action and mechanism.
References
Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D . (1997): Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 154: 805–811
Buchsbaum MS, Wu JC, DeLisi LE, Holcomb HH, Hazlett E, Cooper-Langston K, Kessler R . (1987): Positron emission tomography studies of basal ganglia and somatosensory cortex neuroleptic drug effects: Differences between normal controls and schizophrenic patients. Biol Psychiatry 22: 479–494
Burdett NG, Menon DK, Carpenter TA, Jones JG, Hall LD . (1995): Visualisation of changes in regional cerebral blood flow (rCBF) produced by ketamine using long TE gradient-echo sequences: Preliminary results. Magn Reson Imaging 13: 549–553
Cavazzuti M, Porro CA, Biral GP, Benassi C, Barbieri GC . (1987): Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab 7: 806–811
Friston KJ, Jezzard P, Turner R . (1994a): Analysis of functional MRI time-series. Hum Brain Mapping 1: 153–171
Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC . (1994b): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapping 1: 210–220
Goodman-Gilman A . (1995): The Pharmacological Basis of Therapeutics. New York, McGraw-Hill
Greenamyre JT, Porter RH . (1994): Anatomy and physiology of glutamate in the CNS. Neurology 44: S7–S13
Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA . (1996): Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. A J Psychiatry 153: 41–49
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner D, Heninger GR, Bowers MB, Charney DS . (1994): Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214
Lahti AC, Holcomb HH, Medoff DR, Tamminga CA . (1995a): Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport 6: 869–872
Lahti AC, Koffel B, LaPorte D, Tamminga CA . (1995b): Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13: 9–19
Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A . (1996): NMDA receptor function and human cognition: The effects of ketamine in healthy volunteers. Neuropsychopharmacology 14: 301–307
Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH . (1994): Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540
Porter RH, Greenamyre JT . (1995): Regional variations in the pharmacology of NMDA receptor channel blockers: Implications for therapeutic potential. J Neurochem 64: 614–623
Priestley T, Kemp JA . (1993): Agonist response kinetics of N-methyl-D-aspartate receptors in neurons cultured from rat cerebral cortex and cerebellum: Evidence for receptor heterogeneity. Mol Pharmacol 44: 1252–1257
Talairach J, Tournoux P . (1988): A Co-Planar Stereotaxic Atlas of a Human Brain. Stuttgart, Germany, Thieme-Verlag
Tamminga CA . (1998): Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 12: 21–36
Tamminga CA, Tanimoto K, Kuo S, Chase TN, Contreras PC, Rice KC, Jackson AE, O'Donohue TL . (1987): PCP-induced alterations in cerebral glucose utilization in rat brain: Blockade by metaphit, a PCP-receptor-acylating agent. Synapse 1: 497–504
VanPutten T, Marder SR, Wirshing WC, Aravagiri M, Chabert N . (1991): Neuroleptic plasma levels. Schizophr Bull 17: 197–216
Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J . (1997): Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 7: 25–38
Weissman AD, Dam M, London ED . (1987): Alterations in local cerebral glucose utilization induced by phencyclidine. Brain Res 435: 29–40
Whetsell WO Jr, Shapira NA . (1993): Neuroexcitation, excitotoxicity and human neurological disease. Lab Invest 68: 372–387
White PF, Way WL, Trevor AJ . (1982): Ketamine — its pharmacology and therapeutic uses. Anesthesiology 56: 119–136
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holcomb, H., Lahti, A., Medoff, D. et al. Sequential Regional Cerebral Blood Flow Brain Scans Using PET with H215O Demonstrate Ketamine Actions in CNS Dynamically. Neuropsychopharmacol 25, 165–172 (2001). https://doi.org/10.1016/S0893-133X(01)00229-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(01)00229-9
Keywords
This article is cited by
-
Region- and time- specific effects of ketamine on cerebral blood flow: a randomized controlled trial
Neuropsychopharmacology (2023)
-
The NMDA receptor antagonist ketamine impairs and delays context-dependent decision making in the parietal cortex
Communications Biology (2022)
-
Context-Specific Tolerance and Pharmacological Changes in the Infralimbic Cortex-Nucleus Accumbens Shell Pathway Evoked by Ketamine
Neurochemical Research (2021)
-
Neurometabolic correlates of 6 and 16 weeks of treatment with risperidone in medication-naive first-episode psychosis patients
Translational Psychiatry (2020)
-
Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention
Molecular Psychiatry (2018)