Abstract
The effects of N-[1-(2-benzo[b]thiophenyl)cyclohexyl]- piperidine (BTCP), a phencyclidine derivative that acts as a potent dopamine reuptake inhibitor, were examined on cocaine self-administration in rats. The effects of BTCP (0, 4, 8, 16, and 32 mg/kg, i.p.) on cocaine self-administration were tested against cocaine doses on both the ascending (0.0625 mg/infusion) and descending (0.25 mg/infusion) limb of the dose-response function. BTCP decreased self-administration of the 0.25-mg cocaine dose in a dose-dependent manner. A 16-mg/kg dose of BTCP that strongly suppressed self-administration of the 0.25-mg cocaine dose increased the intake at the 0.0625-mg dose of cocaine. Moreover, cocaine and BTCP pretreatments produced similar patterns of decreases in self-administration of cocaine on the descending limb of the dose-response function. The results suggest that BTCP has cocaine-like actions and produces a leftward shift of the dose-response curve for cocaine self-administration, indicating that the phencyclidine analog may substitute under certain conditions for the reinforcing effects of cocaine in self-administering rats.
Similar content being viewed by others
Main
Cocaine is a highly addictive substance that is abused worldwide (Warner 1993; Higgins 1997). Cocaine also acts as a potent reinforcer in laboratory animals (Pickens and Thompson 1968; Koob 1992; Stolerman 1992; Woolverton and Johnson 1992). It is widely accepted that the addictive and reinforcing actions of cocaine are the result of the drug's ability to block the reuptake of dopamine (DA) by inhibiting the dopamine transporter (DAT) and, thereby, increasing DA neurotransmission (Kuhar 1992; Ritz et al. 1987; Parsons et al. 1998; Woolverton 1992). N-[1-(2-benzo[b]thiophenyl)cyclohexyl] piperidine (BTCP) is a phencyclidine (PCP) derivative that has high affinity for the DAT (Chaudieu et al. 1989; Vignon et al. 1988) but binds to a different site on the transporter than cocaine (Maurice et al. 1991a,b, 1993; Akunne et al. 1994).
BTCP, like cocaine, inhibits DA uptake (Chaudieu et al. 1989; Vignon et al. 1988) and is, in fact, one of the most potent inhibitors of DA reuptake known to date (Chaudieu et al. 1989). Like cocaine, BTCP increases extracellular DA levels in the striatum and the nucleus accumbens (Martin-Fardon et al. 1996a; Maurice et al. 1992), and stimulates locomotor activity in rats (Slimani et al. 1988) and mice (Ilagouma et al. 1993; Koek et al. 1989). Moreover, BTCP has been shown to substitute for cocaine in drug discrimination (Koek et al. 1989) and self-administration studies (French et al. 1995).
While the behavioral and neurochemical effects of acute BTCP administration are similar to those of cocaine (French et al. 1995; Ilagouma et al. 1993; Koek et al. 1989; Martin-Fardon et al. 1996a; Slimani et al. 1988), differences between BTCP and cocaine have been observed with chronic treatment (Martin-Fardon et al. 1996b; Prinssen et al. 1996). In contrast to cocaine, repeated BTCP administration in mice not only failed to produce cocaine-like sensitization of locomotor activity (Prinssen et al. 1996) but resulted in tolerance. Similarly, BTCP attenuated cocaine-induced increases in extracellular DA levels in the striatum, and chronic treatment with BTCP induced a tolerant dopaminergic response to subsequent BTCP challenges (Martin-Fardon et al. 1996b). These data suggest that BTCP can mimic some of the behavioral actions of cocaine, but at the same time may perhaps have a lower abuse potential and produce fewer adverse side effects than cocaine. Such a behavioral profile would identify this new compound as a possible candidate suitable for agonist pharmacotherapy of cocaine addiction (Rothman 1990; Kreek 1997). However, the actions of BTCP are not yet sufficiently well known to support such a possible agonist therapeutic profile. The purpose of the present study was, therefore, to further characterize the effect of BTCP, as well as its interaction with cocaine maintained-behavior in self-administering rats.
MATERIALS AND METHODS
Animals
Male Wistar rats (Beckman Laboratories, The Scripps Research Institute, La Jolla, CA) weighing 200–250 g upon arrival were used. The rats were group-housed (2–3 per cage) in a temperature and humidity controlled vivarium on a 12/12 h light/dark cycle (lights off at 6 P.M.) with ad libitum access to food and water, except during operant training for food reinforcement (see Self-Administration Training). All animals were handled once daily for 5 min during the first week after arrival. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory animals.
Drugs
BTCP was a gift from Dr. Jean-Marc Kamenka (CNRS UPR 1086, ENSCM, Montpellier, France). Cocaine was obtained from the National Institute on Drug Abuse. BTCP was dissolved in sterile physiological saline and administered intraperitoneally (i.p.) at a dose of 1.6 ml/ kg. Cocaine was filtered sterile and dissolved in sterile physiological saline.
Surgery
Rats were prepared with chronic silastic jugular catheters under halothane anesthesia (1.5–2.0 %) as previously described (Caine et al. 1993). All the animals were treated with antibiotic (Timentin® 10 mg, i.v.) during the first four days post-surgery. Catheter patency was maintained by flushing with 0.1 ml of sterile heparin/saline solution (30 USP units/ml) before and after each self-administration session. Rats with compromised catheters were implanted with a new catheter in the controlateral jugular vein when possible, or excluded from the experiment.
Behavioral Testing Apparatus
Self-administration training and testing was conducted in standard 29 × 24 × 19.5 cm (L × W × H) operant chambers located inside ventilated sound-attenuating cubicles (BRS/LVE Inc., Laurel, MD). The chambers were equipped with a retractable lever positioned 4 cm above the grid floor on the right side of the front panel and a white cue light located 6 cm above the lever. A counterbalancing arm fitted with a single-channel liquid swivel (Model 375; Instech Labs, Plymouth Meeting, PA) was suspended above the operant chamber. The inlet of the liquid swivel was connected with polyethylene tubing to a 10 ml syringe containing the drug solution, and the liquid swivel outlet was connected via a cannula connector (C313G-5UP; Plastics One, Roanoke, VA) to the chronic jugular catheter on the rat's back. Intravenous infusions were administered by activation of a syringe pump (Razel Scientific Instrument, Stanford, CT) located outside the sound attenuating boxes. Behavioral testing protocols and data collection was controlled by an IBM-compatible microcomputer.
Self-Administration Training
Prior to implantation of intravenous catheters, rats were food deprived for 24 hr and then maintained on a restricted diet (20 g standard laboratory chow per day). During this period, the animals were trained to lever-press for 45 mg food pellets (PJ Noyes Company, Inc, Lancaster, NH) on a fixed-ratio 5 (FR 5), time-out 20 sec (TO 20) schedule. Operant training for food reinforcers was continued until a criterion of at least 50 completed ratio requirements per 30 min session over three consecutive days was reached. All house lights and stimulus lights were off during the food and cocaine self-administration sessions. Completion of each ratio requirement resulted in delivery of a food pellet and illumination of the cue light above the lever for 20 sec. The animals were then returned to ad libitum food availability and, 2–3 days later, surgically implanted with chronic jugular catheters.
One week after recovery from surgery, cocaine self-administration began in daily 3-hr sessions conducted six days per week. Cocaine self-administration sessions were initiated by administration of two non-contingent intravenous cocaine infusions (0.25 mg/0.1 ml/infusion, delivered over 4 sec). Each infusion was followed by a 20-sec period during which time the white cue light above the lever was illuminated. Subsequently, the right lever was extended into the operant chamber at which time cocaine (at the training dose of 0.25 mg/0.1 ml) was made available on a FR 5 schedule of reinforcement. After each infusion, the lever remained inactive for 20 sec to avoid accidental overdosing. This time-out period was signaled by illumination of the white cue light. Testing began once all rats reached stable baselines of responding, defined as no more than 10% variation in the total number of drug infusions over three consecutive days. The animals weighed 380.7 ± 11.2 g (n = 7) at the beginning of cocaine selfadministration training, and 559.6 ± 26.5 g (n = 5) after completion of all behavioral testing.
Behavioral Testing Procedures
All experiments were conducted in 3 hr limited-access self-administration sessions on an FR 5 schedule of reinforcement, and all sessions were initiated by delivery of two non-contingent cocaine injections (0.25 mg/infusion). Before the beginning of tests of BTCP effects on cocaine self-administration, dose-effect functions for cocaine-maintained responding were established by determining the total number of cocaine infusions per 3 hr session obtained by rats (n = 7) during self-administration of each of four different cocaine concentrations (0.0625, 0.125, 0.25, and 0.5 mg/infusion). To control for order effects, animals were tested with the four different cocaine doses in a random sequence. Tests with all drug doses were conducted only when responding for cocaine was stable as defined by a criterion of less than ± 10% variation of the total number of infusions over three consecutive self-administration sessions. Additionally, in a replication of these dose-effect determinations, the effects of saline administration (1.6 ml/kg, administered 10 min before the beginning of selfadministration sessions) were examined in order to control for any disruptive effects associated with the drug injection procedure per se in the subsequent tests.
After the completion of the cocaine dose-response tests, the rats (n = 7) were again given daily access to cocaine until stable baselines of cocaine self-administration (at 0.25 mg/infusion) were reestablished. Subsequently, the effects of four doses of BTCP (4, 8, 16, 32 mg/kg, i.p.) and its vehicle (saline, 1.6 ml/kg) on self-administration of the training dose of cocaine (0.25 mg/infusion) were determined. BTCP was administered 10 min before the beginning of 3 hr self-administration sessions. Each animal was tested with all doses of BTCP in random order. Each animal was tested once with each dose of BTCP.
After reestablishing stable baselines of responding for cocaine, the effect of pretreatment with cocaine (32 mg/kg, i.p., administered 10 min before onset of testing) on selfadministration of the training dose of cocaine was examined. This dose of cocaine was selected in view of earlier findings documenting that BTCP is more potent than cocaine with regard to its ability to elevate extracellular dopamine levels in the striatum and, specifically, a given dose of cocaine is equivalent to one half of the corresponding dose of BTCP (Martin-Fardon et al. 1996a). Based on these previous observations, a 32 mg/kg dose of cocaine was considered to be equivalent to a 16 mg/kg dose of BTCP.
For the last experiment, the dose of cocaine available in daily self-administration sessions was lowered to 0.0625 mg/infusion (a sub-threshold dose for maintaining cocaine-reinforced responding). Once stable baselines of responding were established, the effects of BTCP vehicle (saline, 1.6 ml/kg) and BTCP (16 mg/kg, i.p.) pretreatment on intravenous self-administration of this dose of cocaine (0.0625 mg/infusion) were established on two successive days.
Effect of BTCP on Water-Reinforced Behavior
To control for nonspecific inhibitory behavioral effects of BTCP, the effects of this compound on responding for water were examined. Twelve male Wistar rats were placed on a 21 hour/day water restriction schedule. The animals were then trained to lever-press on an FR 1 schedule for 0.1 ml water in daily 30 min sessions in the same operant chamber used for all other experiments except that the food hopper was replaced by a drinking well (see Self-Administration Training). Water selfadministration sessions were conducted once per day during five consecutive days per week. Once a stable baseline of responding for water was established (± 15 % over three consecutive sessions), the animals were divided into two groups. Group 1 was treated with saline (1.6 ml/kg, i.p.), and Group 2 with BTCP (16 mg/kg, i.p.), 10 min before the beginning of water self-administration sessions.
Statistical Analysis
Differences in the effects of drug treatments on the total number of infusions per 3 hr session were analyzed by planned contrasts among means. Dose-dependent differences in the cumulative number of infusions were analyzed by two-way within-subjects ANOVA followed by analysis of Simple Effects and Newman Keuls post-hoc tests. Water-reinforced responses were analyzed by a mixed-factorial ANOVA.
RESULTS
Dose-Effect Functions for Cocaine Self-Administration
Self-administration of the four different unit doses of cocaine produced an inverted U-shaped dose-effect curve with highest rates of responding maintained by the 0.125 mg/infusion dose. The mean (± SEM) total number of cocaine infusions/session at the four different doses were 12.6 ± 2.3 (0.0625 mg/infusion), 99.1 ± 5.5 (0.125 mg/infusion), 51.1 ± 1.3 (0.25 mg/infusion), and 30.0 ± 1.7 (0.5 mg/infusion). The training dose of 0.25 mg/infusion was located on the descending limb of the dose-effect function and the lowest dose of cocaine (0.0625 mg/infusion) did not maintain behavior. Administration of saline 10 min before self-administration sessions had no effect on cocaine self-administration at any dose and the mean (±SEM) total number of infusions at the different cocaine doses were 11.6 ± 1.8 (0.0625 mg/infusion), 100.7 ± 5.3 (0.125 mg/infusion), 53.7 ± 1.7 (0.25 mg/infusion), and 31.3 ± 1.3 (0.5 mg/infusion).
Effects of BTCP Pretreatment on Self-Administration of a Cocaine Dose on the Descending Limb of the Dose-Effect Function
Mean (± SEM) baseline responding (51.1 ± 1.3) for the cocaine training dose (0.25 mg/infusion) after BTCP vehicle injection (saline 1.6 ml/kg) remained unaltered compared to self-administration performance in the preceding experiment (52.6 ± 2.1) (Figure 1A). BTCP pretreatments dose-dependently decreased the self-administration of a cocaine dose on the descending limb of the cocaine dose-effect function (0.25 mg/infusion) (Figure 1A). Compared to the vehicle condition, cocaine intake was significantly reduced after BTCP doses of 8 mg/kg [F(1,6) = 10.4, p < .05], 16 mg/kg [F(1,6) = 84.7, p < .001], and 32 mg/kg [F(1,6) = 51.1 p < .001], but not after the 4 mg/kg dose of BTCP.
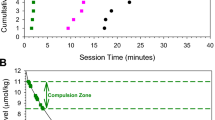
Effects of intraperitoneal BTCP or cocaine pretreatment on self-administration of a cocaine dose (0.25 mg/infusion) on the descending limb of the dose-effect function. (A) Effects of saline and BTCP pretreatments on the total number of infusions obtained during 3 h sessions (* p < .05; *** p < .001; significantly different from Saline). (B) Effects of saline and BTCP pretreatment on the cumulative number of cocaine infusions (see Results for statistical comparisons). (C) Effect of saline and cocaine (32 mg/kg, i.p.) pretreatment on the total number of infusions recorded during 3 h self-administration sessions (*** p < .001). (D) Effect of saline and cocaine pretreatment on the cumulative number of cocaine infusions (see Results for statistical comparisons)
Examination of the temporal profile of BTCP effects on responding for cocaine revealed that BTCP pretreatment delayed the initiation of self-administration and decreased the number of infusions self-administered per unit time, once self-administration was initiated. As illustrated by the slopes of the cumulative response plots shown in Figure 1B, both of these effects were dose-dependent. Overall, differences in response patterns generated by the vehicle vs. BTCP pretreatments were confirmed by a significant effect for drug doses [F(4,14) = 54.72; p < .00001] and a significant interaction between these factors [F(58,408) = 35.55; p < .00001]. Newman Keuls tests further confirmed significant differences from saline pretreatment for all (except the 4 mg/kg) BTCP doses (p < .01), as well as significant dose-dependent differences among the effects of the four BTCP doses (4 vs. 8 mg/kg, p < .05; 8 vs. 16 mg/kg, p < .01; 16 vs. 32 mg/kg, p < .01). In addition, simple effects analysis revealed that the effects of BTCP pretreatments were significant at each (p < .0001) session interval.
Effects of Cocaine Pretreatment on Self-Administration of a Cocaine Dose on the Descending Limb of the Dose-Effect Function
One animal died during this phase of the experiment reducing the sample size to six rats. There were no differences in baseline cocaine self-administration (0.25 mg/infusion after saline pretreatment) measured before (50.5 ± 1.4) and after (54.0 ± 1.8) completion of the BTCP dose-effect experiment in the remaining rats. Pretreatment with cocaine (32 mg/kg, i.p.) produced a significant decrease [F(1,5) = 82.7, p < .001] in the number of self-administered cocaine infusions (34.5 ± 1.3), whereas cocaine vehicle pretreatment (saline) had no effect (50.7 ± 1.0) on responding for cocaine (Figure 1C).
Analysis of the temporal profile of the cocaine pretreatment effects indicated a delay in the onset of intravenous self-administration and a decrease in the number of infusions per unit time (Figure 1D). These behavioral effects were nearly identical to those produced by BTCP at the 16 mg/kg dose in the preceding experiment. The decrease in the cumulative number of cocaine infusions was confirmed by a significant main effect for drug pretreatment [F(1,5) = 107.8, p < .001] and a significant interaction between drug pretreatment and responses over time [F(17,85) = 32.6, p < .001]. Subsequent analysis of Simple Effects indicated that the cumulative number of infusions was lower compared to that after saline pretreatment at all time points (10 to 180 min, p < .001).
Effects of BTCP Pretreatment on Self-Administration of a Cocaine Dose on the Ascending Limb of the Dose-Effect Function
One rat was eliminated during this phase of the experiment because of loss of catheter patency, reducing the sample size to five animals. This experiment examined the effects of BTCP on the self-administration of a cocaine dose on the ascending limb of the dose effect-function (0.0625 mg/infusion). Pretreatment with BTCP (16 mg/kg, i.p.) but not its vehicle (saline) produced a significant increase [F(1,4) = 14.7, p < .05] in the total number of self-infusions of the 0.0625 mg cocaine dose (Saline: 6.2 ± 0.6, BTCP: 102.4 ± 25.1) (Figure 2A).
. Effect of BTCP (16 mg/kg, i.p.) pretreatment on self-administration of a cocaine dose (0.0625 mg/infusion) on the ascending limb of the dose-effect function. (A) Effect of BTCP on the total number of infusions self-administered during a 3 h session (* p < .05). (B) Effect of BTCP on the cumulative number of cocaine infusions (see Results for statistical comparisons)
The difference between the cumulative number of cocaine infusions in BTCP vs. saline pretreated animals (Figure 2B) was confirmed by a significant main effect for drug treatment [F(1,4) = 11.9, p < .05] and a significant interaction between drug treatments and the cumulative number of cocaine infusions [F(17,68) = 14.5, p < .001]. Twenty minutes after the beginning of the session, BTCP-treated animals began to lever-press and maintained stable levels of responding until the end of the self-administration period. Simple Effects analysis indicated that the BTCP-induced increase in cocaine intake was significant between 80 and 180 min of the self-administration session (p < .05).
Effect of BTCP on Saline Self-Administration
To control for the possible contribution of priming effects of BTCP to the increased self-administration of the low cocaine dose, the effects of BTCP were examined after saline substitution. For this purpose, a group of four additional rats was trained to self-administer cocaine (0.25 mg/infusion) in a daily-3 hr session (see Behavioral Testing Procedures). Once the rats developed stable cocaine intake (± 10% over three consecutive sessions) the cocaine solution was replaced with saline. Saline sessions were conducted until extinction responses stabilized. At this time the effect of BTCP (16 mg/kg, i.p.) (and its vehicle) was tested on two successive days.
Mean (± SEM) baseline responding (48.3 ± 4.5; n = 4) for the cocaine training dose (0.25 mg/infusion) was similar to that observed during the cocaine dose-effect determination in the previously tested animals (see in the Results section: Dose-Effect Functions for Cocaine Self-Administration). Substitution of saline produced an extinction of self-administration with a mean total number of 4.4 ± 1.7 infusions per session over 4.0 ± 0.6 saline substitution days. Pretreatment with BTCP (16 mg/kg, i.p.) or vehicle did not modify the self-administration of saline [BTCP: 12.0 ± 2.8; vehicle: 6.8 ± 1.9; F(2,6) = 1.5; NS].
Effect of BTCP on Water-Seeking Behavior
The baseline number of responses for water before the drug treatments was identical in the two groups of animals subsequently tested with BTCP (138.0 ± 12.2; n = 6) vs. Saline (134.5 ± 14.4; n = 6). Neither saline (145.3 ± 18.0) nor BTCP (124.8 ± 26.5) pretreatment altered responding for water compared to baseline levels [F(1,10) = 0.007; NS].
DISCUSSION
Pretreatment with the potent DA uptake inhibitor BTCP dose-dependently decreased self-administration of a cocaine dose on the descending limb of the cocaine dose-effect function (0.25 mg/infusion). In addition, BTCP increased the intake of a lower cocaine dose (0.0625 mg/infusion) that by itself did not maintain reliable self-administration. In fact, BTCP pretreatment resulted in maintenance of self-administration by this cocaine dose throughout the session, but did not modify the self-administration of saline. Thus, BTCP decreased cocaine self-administration on the descending limb of the cocaine dose-response curve while increasing cocaine intake on the ascending limb (Figure 3). These observations suggest that BTCP produces a leftward shift in the cocaine dose-response curve.
Baseline levels of cocaine-reinforced responding at the training dose (0.25 mg/infusion) remained essentially unaltered throughout the entire experiment in spite of increases in the animals' body weight. This observation is in agreement with previous findings showing that under conditions of limited daily access to cocaine, rats maintain a highly constant level of cocaine intake over long periods of time, and resume responding at the same levels after periods of forced abstinence (Ahmed and Koob 1998). This stability of cocaine intake has been interpreted as reflecting a constant “hedonic set point” in limited-access cocaine self-administration models (Ahmed and Koob 1998).
Previous studies in rats have shown that pretreatment with dopamine agonists can decrease self-administration of amphetamine and cocaine doses on the descending limb of the dose-effect function for these stimulants (Tella 1995; Yokel and Wise 1978). Similarly, pretreatment with cocaine decreased subsequent self-administration of cocaine in monkeys (Herling et al. 1979). Consistent with these reports, cocaine pretreatment lowered the amount of cocaine self-administered by rats in the present study. Interestingly, pretreatment with BTCP produced similar behavioral effects as cocaine pretreatement (Figure 1), but with greater potency.
Comparison of the effects of equimolar doses of BTCP (32 mg/kg ∼59.6 mM) and cocaine (32 mg/kg ∼59.0 mM) indicates that BTCP pretreatment resulted in a net reduction of cocaine self-administration that was approximately 30% greater than that produced by cocaine pretreatment (Figures 1A and 1C). This observation extends previous findings showing that BTCP produces greater locomotor activation as well as greater increases in striatal dopamine efflux than equivalent doses of cocaine (Martin-Fardon et al. 1996a, b; Prinssen et al. 1996). However, it was recently shown that the self-administration of equimolar concentrations of BTCP or cocaine on a progressive-ratio schedule produces identical breaking points (French et al. 1995), suggesting that BTCP and cocaine have equal reinforcing potency. This discrepancy may be related to differences in the test paradigms employed to assess the reinforcing actions of the two drugs (i.e., self-administration of BTCP vs. BTCP “pre-loading” effects on cocaine self-administration).
The apparent greater behavioral potency of BTCP compared to cocaine in modifying self-administration of cocaine in the present study may, therefore, reflect specific interactions of the phencyclidine analog with cocaine, rather than a greater intrinsic reinforcing potency of BTCP itself. A possible explanation for the greater apparent potency of BTCP over cocaine may be differences in elimination profiles and generation of active metabolites between the two compounds. Cocaine's main metabolite, benzoylecgonine, has little pharmacological activity (for review see Benowitz 1992). In contrast, BTCP has two active metabolites with high affinity for the DA transporter, 1-[1-(2-benzo[b]thiopheneyl)cyclohexyl] piperidin-4-ol and 1-[1-(2-benzo[b]thiopheneyl) cyclohexyl]piperidin-3-ol (Deleuze-Masquefa et al. 1997). The greater delay in the onset of responding and the decrease in cocaine intake per 10 minute interval induced by BTCP compared to cocaine may, therefore, be attributable to an action of these active metabolites (see Figures 1B and 1D for comparison).
Under limited-access schedules of cocaine self-administration, rats exhibit a very stable and reproducible pattern of behavior that is characterized by an initial “loading phase” followed by the development of a regular response pattern and dose-titration (Koob et al. 1987; Roberts et al. 1980; Roberts and Koob 1982). During this latter phase, the length of interinjection intervals is highly constant with the number of injections per unit time inversely related to the dose of cocaine. The high frequency of cocaine intake during the loading phase as well as the behavioral titration to a given unit dose of the drug suggests that animals regulate cocaine intake in a manner that induces and maintains a specific level of dopaminergic activation (Wise et al. 1995; Pettit and Justice 1991). BTCP, administered intraperitoneally 10 minutes before the beginning of the sessions, dose-dependently delayed the onset of intravenous cocaine self-administration and reduced the number of responses during the “loading phase” once animals began responding for cocaine compared to rats pretreated with intraperitoneal saline. After the end of the loading phase, BTCP continued to produce dose-dependent reductions in cocaine intake by increasing the length of interinjection intervals. Pretreatment with cocaine (32 mg/kg) produced effects that were essentially identical to the 16-mg/kg dose of BTCP (see Figures 1A and 1C).
The compensatory delays in responding during the loading phase and increases in interinjection intervals during the maintenance phase resemble dose-titration changes in self-administration induced by increasing the unit dose of cocaine. This observation as well as the similarity between the effects of BTCP and cocaine pretreatments suggests that BTCP enhanced the behavioral actions of intravenous cocaine by exerting effects similar to those of higher unit doses of cocaine. This conclusion is further supported by similarities in dopaminergic activation produced by cocaine and BTCP established in previous work. Both cocaine and BTCP increase extracellular dopamine levels 10–20 minutes after intraperitoneal administration with maximum effects at 60 minutes and a return to baseline levels approximately 2 hours after administration, albeit with a somewhat more shallow and sustained temporal profile of BTCP effects compared to cocaine (Martin-Fardon et al. 1996a). In the present study, rats were injected with BTCP or cocaine 10 min before the sessions which began by administration of two non-contingent cocaine infusions. Thus, rats were subjected to the additive effects of the BTCP or cocaine pretreatment and the two intravenous cocaine infusions. The behavioral changes induced by these treatments seemed to parallel the time course of their neurochemical effects. This was particularly evident at the higher doses of BTCP (16 and 32 mg/kg) and after cocaine (32 mg/kg) pretreatments that delayed the onset of cocaine self-administration in a manner that seemed to correspond to the decline in the effects of BTCP or cocaine pretreatments on accumbal extracellular dopamine levels.
While BTCP lowered self-administration of a cocaine dose located on the descending limb of the dose-effect function (i.e., the 0.25 mg/infusion training dose), BTCP (16 mg/kg) produced a robust increase in self-administration of a cocaine dose (0.0625 mg/infusion) on the ascending limb of the dose-response curve (Figure 2) that did not maintain responding by itself. As shown in Figure 2B, BTCP-pretreated animals began responding after a delay of 10 minutes and developed stable rates of cocaine self-administration which were maintained for the remainder of the session. This observation is consistent with the interpretation that the 16-mg/kg cocaine dose effectively maintained behavior in BTCP-pretreated animals. Since BTCP did not increase the self-administration of saline, the BTCP-induced maintenance of responding by a non-reinforcing dose of cocaine cannot be attributed to a priming effect by BTCP. Therefore, the present behavioral results suggest that BTCP initially substituted for cocaine and inhibited responding, while exerting additive effects to those of the low intravenous cocaine dose at later stages and, thereby, maintaining reliable self-administration of this subthreshold dose of cocaine.
The delay in the onset of cocaine self-administration induced by BTCP seems to be specific to cocaine-reinforced responding rather than being the result of non-specific behavioral inhibition. This conclusion is supported by the lack of suppressant effects of BTCP (at the 16-mg/kg dose) on water-reinforced responding. However, Koek et al. (1989) reported that a BTCP dose as low as 10 mg/kg (i.p.) suppressed responding for food in a drug discrimination paradigm. In the latter experiment, BTCP was injected 15 min before the beginning of the session and the behavior was monitored for another 15 min. It is possible that the suppression of lever-pressing for food observed by Koek et al. (1989) might be an expression BTCP's anorexic effects (Duterte-Boucher et al. 1990). Given that the behavioral and neurochemical effects of a 32 mg/kg cocaine dose seem to be equivalent to the16-mg/kg BTCP dose, it seems reasonable to assume that the lack of responding during the early phase of the session after 32 mg/kg cocaine was similarly not the result of general behavioral suppression (stereotypy), but rather reflected a compensatory delay in responding in response to the high intraperitoneal cocaine dose.
Cocaine self-administration is modified not only by pharmacological manipulation of dopaminergic transmission, but also by ligands acting at glutamate receptors (De Vries et al. 1998; Ranaldi et al. 1996; Schenk et al. 1993). In particular, administration of the non-competitive N-methyl-D-aspartate (NMDA) antagonist MK-801 can enhance the reinforcing effects of cocaine (Ranaldi et al. 1996), and reinstate cocaine seeking behavior after extinction (De Vries et al. 1998). Moreover, there is evidence that the abuse liability of NMDA antagonists is not entirely DA dependent (e.g., Carlezon and Wise 1996; Nicholson et al. 1997, 1998). Since BTCP is a phencyclidine derivative and has some affinity for the PCP receptor, some of its behavioral actions may be the result of an action at the NMDA receptor. However, while BTCP is a PCP derivative, its predominant pharmacological action is a potent and highly selective inhibition of DA reuptake (Chaudieu et al. 1989; Vignon et al. 1988), but has only low affinity for the PCP receptor itself. Moreover, in contrast to the non-competitive NMDA antagonists MK-801 and PCP, BTCP does not antagonize NMDA-induced convulsions in mice (Koek et al. 1989). Thus, the pharmacological mechanism of action of BTCP does not seem to be linked to possible NMDA effects as might be inferred from this compound's chemical structure. Consequently, it appears unlikely that the modification of cocaine reinforcement by BTCP in the present study can be attributed to some residual non-competitive NMDA antagonist action rather than a direct effect on DA uptake.
The reinforcing actions of cocaine are mediated by its action to inhibit DA reuptake (Ritz et al. 1987), but cocaine also inhibits the reuptake of DA, noradrenaline (NA) and serotonin (5-HT) and these effects may contribute to, or modulate the reinforcing behavioral consequences of cocaine administration. The potency of BTCP for inhibition of 5-HT and NA transporters is about the same as that for DA reuptake (Lebel et al. 1994), but there is much evidence that it is specifically the inhibition of DA reuptake that accounts for the behavioral cocaine-like effects of BTCP (Giros et al. 1996; Roberts et al. 1999; Silvia et al. 1997). BTCP has also been reported to interact with the NA system by inhibiting the electrical activity of NA neurons in anesthetized rats (Chergui et al. 1992). However, in a recent study, PCP-induced place preference in mice was blocked by coadministration of the DA synthesis inhibitor α-methyl-ρ-tyrosine or 6-hydoxydopamine lesions of DA neurons but not by lesion of NA neurons using N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine, a NA neurotoxin, nor by risanterin, a 5-HT2 receptor antagonist (Noda et al. 1998). These observations suggest that PCP-induced place preference is mediated by dopaminergic rather than serotonergic or noradrenergic systems. Therefore, neither a possible action of BTCP on noradrenergic systems, NMDA receptors or 5-HT receptors would seem to account for the effects of BTCP on the cocaine self-administration in the present study.
Together, these findings indicate that BTCP shifted the dose-response function for cocaine self-administration to the left. The BTCP-induced leftward shift is similar to that induced by higher unit doses of cocaine and, therefore, suggestive of an enhancement of the reinforcing actions of cocaine. BTCP inhibits dopamine reuptake and, in this respect, exerts similar, albeit more potent, pharmacological actions as cocaine (Chaudieu et al. 1989; Vignon et al. 1988). Moreover, cocaine and BTCP produced identical decreases in self-administration of a cocaine dose on the descending limb of the dose-response function, confirming earlier findings that BTCP and cocaine have similar behavioral actions (French et al. 1995; Koek et al. 1989; Martin-Fardon et al. 1996a). These observations are consistent with the hypothesis that BTCP and cocaine interact with the same neurochemical system and that inhibition of dopamine reuptake by BTCP is the mechanism of action underlying the enhancement of cocaine reinforcement by this phencyclidine derivative.
One strategy for the treatment of cocaine addiction focuses on the use of compounds that substitute for the actions of the primary drug of abuse but have a longer duration of action paired with lower intrinsic abuse potential and toxic side effects (Rothman 1990; Kreek 1997). In the case of cocaine, therapeutic agents with indirect agonist properties (e.g., uptake inhibitors) are likely to be more effective than direct dopamine receptor agonists because of emetic and other side effects associated with drugs of the latter class. The present results indicate that BTCP can substitute for the reinforcing effects of cocaine and does so with greater potency than cocaine. However, whether the behavioral profile of BTCP identified by the results qualifies this drug as a possible agonist substitution drug remains unclear. Indeed, a single intraperitoneal injection of BTCP before access to a subthreshold dose of cocaine rendered this dose of cocaine reinforcing which may reflect a short-acting pharmacokinetic profile of BTCP. However, the route of administration (i.e., intraperitoneal vs. oral) as well as the duration of action and the examination of the effects of single versus chronic administration are other important issues that require systematic exploration. Thus, further studies, particularly of BTCP's duration of action and its intrinsic abuse liability, will be essential to more conclusively assess its potential as an agonist therapeutic compound for cocaine addiction.
References
Ahmed SH, Koob GF . (1998): Transition from moderate to excessive drug intake: Change in hedonic set point. Science 282: 298–300
Akunne HC, Dersch CM, Cadet JL, Baumann MH, Char GU, Partilla JS, De Costa BR, Rice KC, Carroll FI, Rothman RB . (1994): Studies of the biogenic amine transporters. III. Demonstration of two binding sites for [3H]GBR-12935 and [3H]BTCP in caudate membranes. J Pharmacol Exp Ther 268: 1462–1475
Benowitz NL . (1992): How toxic is cocaine? Ciba Found Symp 166: 125–143
Caine SB, Lintz R, Koob GF . (1993): Intravenous drug self-administration techniques in animals. In Sahgal A (ed), Behavioural Neuroscience: A Practical Approach. Vol. 2. New York, Oxford University Press, pp 117–143
Carlezon WA, Wise RA . (1996): Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology 128: 413–420
Chaudieu I, Vignon J, Chicheportiche M, Kamenka JM, Trouiller G, Chicheportiche R . (1989): Role of the aromatic group in the inhibition of phencyclidine binding and dopamine uptake by PCP analogs. Pharmacol Biochem Behav 32: 699–705
Chergui K, Akaoka H, Brunet JL, Charlety PJ, Saunier CF, Buda M, Privat A, Vignon J, Kamenka JM, Chouvet G . (1992): Inhibition of locus coeruleus neurons by the phencyclidine analog, N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine: Evidence for potent indirect adrenoceptor agonist properties. Eur J Pharmacol 219: 169–172
Deleuze-Masquefa C, Michaud M, Vignon J, Kamenka JM . (1997): 1-[1-(2-benzo(b)thiopheneyl)cyclohexyl]piperidine hydrochloride (BTCP) yields two active primary metabolites in vitro: Synthesis, identification from rat liver microsome extracts, and affinity for the neuronal dopamine transporter. J Med Chem 40: 4019–4025
De Vries TJ, Schoffelmeer ANM, Binnekade R, Mulder AH, Vanderschuren LJMJ . (1998): MK-801 reinstates drug-seeking behaviour in cocaine-trained rats. NeuroReport 9: 637–640
Duterte-Boucher D, Kamenka JM, Costentin J . (1990): Comparison of the effects of three indirect dopamine agonists, GK 13, GBR 12783 and dexamphetamine on behavioural tests involving central catecholaminergic transmissions. Psychopharmacology 101: 344–353
French ED, Lopez M, Peper S, Kamenka JM, Roberts DCS . (1995): A comparison of the reinforcing efficacy of PCP, the PCP derivatives TCP and BTCP, and cocaine using a progressive ratio schedule in the rat. Behav Pharmacol 6: 223–228
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG . (1996): Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379: 606–612
Herling S, Downs DA, Woods JH . (1979): Cocaine, d-amphetamine and pentobarbital effects on responding maintained by food or cocaine in rhesus monkeys. Psychopharmacology 64: 261–269
Higgins ST . (1997): The influence of alternative reinforcers on cocaine use and abuse: A brief review. Pharmacol Biochem Behav 57: 419–427
Ilagouma AT, Maurice T, Duterte-Boucher D, Coderc E, Vignon J, Costentin J, Kamenka JM . (1993): Arylcyclohexylamines derived from BTCP are potent indirect catecholamine agonists. Eur J Med Chem 28: 377–385
Koek W, Colpaert FC, Woods JH, Kamenka JM . (1989): The phencyclidine (PCP) analog N-[1-(2-Benzo(b)Thiophenyl)Cyclohexyl]Piperidine shares cocaine-like but not other characteristic behavioral effects with PCP, ketamine and MK-801. J Pharmacol Exp Ther 250: 1019–1027
Koob GF . (1992): Drugs of abuse: Anatomy, pharmacology and a function of reward pathways. Trends Pharmacol Sci 13: 177–184
Koob GF, Vaccarino FJ, Amalric M, Swerdlow NR . (1987): Neural substrates for cocaine and opiate reinforcement. In Fisher S, Raskin A, Uhlenhuth EH (eds), Cocaine: Clinical and Biobehavioral Aspects. New York, Oxford University Press, pp 80–108
Kreek MJ . (1997): Opiate and cocaine addictions: Challenge for pharmacotherapies. Pharmacol Biohem Behav 57: 551–569
Kuhar MJ . (1992): Molecular pharmacology of cocaine: A dopamine hypothesis and its implications. Ciba Found Symp 166: 81–89
Lebel LA, Nowakowski JT, Macor JE, Fox CB, Koe BK . (1994): Dopamine uptake inhibitor activity of a novel tryptamine 5-HT-1 receptor ligands. Drug Dev Res 33: 413–421
Martin-Fardon R, Arnaud M, Rousseau E, Kamenka JM, Privat A, Vignon J . (1996a): N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine (BTCP) and cocaine induce similar effects on striatal dopamine: A microdialysis study in freely moving rats. Neurosci Lett 211: 179–182
Martin-Fardon R, Kamenka JM, Koek W, Privat A, Vignon J . (1996b): Effect of chronic and cross treatments with N-[1-(2-benzo[b]thiophenyl)cyclohexyl]piperidine (BTCP) and cocaine on extracellular dopamine concentration in rat striatum. Soc Neurosci Abstr 22: 932
Maurice T, Barbanel G, Kamenka JM, Vignon J . (1991a): Modulation by dopamine of [3H]N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine ([3H]BTCP, a phencyclidine derivative) binding to the dopamine uptake complex. Neuropharmacology 30: 591–598
Maurice T, Barbanel G, Vignon J . (1993): Endogenous dopamine differently affects N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine and cocaine binding to the dopamine uptake complex. Gen Pharmacol 24: 191–194
Maurice T, Roman FJ, Pascaud X, Kamenka JM, Junien JL . (1992): Regional differences in the effect of N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine (BTCP) on extracellular dopamine levels: An in vivo microdialysis study. Neurosci Lett 138: 63–66
Maurice T, Vignon J, Kamenka JM, Chicheportiche R . (1991b): Differential interaction of phencyclidine-like drugs with the dopamine uptake complex in vivo. J Neurochem 56: 553–569
Nicholson KL, Jones HE, Balster RL . (1997): Evaluation of the reinforcing and discriminative stimulus effects of the N-methyl-D-aspartate competitive antagonist NPC 17742 in rhesus monkeys. Behav Pharmacol 8: 396–407
Nicholson KL, Jones HE, Balster RL . (1998): Evaluation of the reinforcing and discriminative stimulus properties of the low-affinity N-methyl-D-aspartate channel blocker memantine. Behav Pharmacol 9: 231–243
Noda Y, Miyamato Y, Mamiya T, Kamei H, Furukawa H, Nabeshima T . (1998): Involvement of dopaminergic system in phencyclidine-induced place preference in mice pretreated with phencyclidine repeatedly. J Pharmacol Exp Therap 286: 44–51
Parsons LH, Kerr TM, Weiss F . (1998): Simple microbore high-performance liquid chromatographic method for the determination of dopamine and cocaine from a single in vivo brain microdialysis sample. J Chromatogr B Biomed Sci Appl 709: 35–45
Pettit HO, Justice JB Jr . (1991): Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res 539: 94–102
Pickens R, Thompson T . (1968): Cocaine-reinforced behavior in rats: Effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther 161: 122–129
Prinssen EPM, Kleven MS, Vignon J, Kamenka JM, Koek W . (1996): Effects of repeated administration of N-[1-2-benzo(b)thiophenyl)cyclohexyl]piperidine and cocaine on locomotor activity in C57BL/6 mice. J Pharmacol Exp Ther 276: 904–911
Ranaldi R, French E, Roberts DCS . (1996): Systemic pretreatment with MK-801 (dizocilpine) increases breaking points for self-administration of cocaine on a pogressive-ratio schedule in rats. Psychopharmacology 128: 83–88
Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ . (1987): Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237: 1219–1223
Roberts DCS, Koob GF . (1982): Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav 17: 901–904
Roberts DCS, Koob GF, Klonoff P, Fibiger HC . (1980): Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 12: 781–787
Roberts DCS, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H . (1999): Self-administration of cocaine analogs by rats. Psychopharmacology 144: 389–397
Rothman RB . (1990): High affinity dopamine reuptake inhibitors as potential cocaine antagonists: A strategy for drug development. Life Sci 46: PL17–PL21
Schenk S, Valadez A, Worley CM, McNamara C . (1993): Blockade of the acquisition of cocaine self-administration by the NMDA antagonist MK-801 (dizocilpine). Behav Pharmacol 4: 652–659
Silvia CP, Jaber M, King GR, Ellinwood EH, Caron MG . (1997): Cocaine and amphetamine elicit differential effects in rats with unilateral injection of dopamine transporter antisense oligodeoxynucleotides. Neuroscience 76: 737–747
Slimani N, Boucher D, Bonnet JJ, Kamenka JM, Costentin J . (1988): Neurochemical and behavioral evidence for a central indirect dopaminergic activity of GK13, a phencyclidine derivative. In Domino EF, Kamenka JM (eds), Sigma and Phencyclidine-Like Compounds as Molecular Probes in Biology. Ann Arbor, NPP Books, pp 511–520
Stolerman I . (1992): Drugs of abuse: Behavioural principles, methods and terms. Trends Pharmacol Sci 13: 170–176
Tella SR . (1995): Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav 51: 687–692
Vignon J, Pinet V, Cerruti C, Kamenka JM, Chicheportiche R . (1988): [3H]N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine ([3H]BTCP): A new phencyclidine analog selective for the dopamine uptake complex. Eur J Pharmacol 148: 427–436
Warner EA . (1993): Cocaine abuse. Ann Intern Med 119: 226–235
Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB Jr . (1995): Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology 120: 10–20
Woolverton WL . (1992): Determinants of cocaine self-administration by laboratory animals. Ciba Found Symp 166: 149–161
Woolverton WL, Johnson KM . (1992): Neurobiology of cocaine abuse. Trends Pharmacol Sci 13: 193–200
Yokel RA, Wise RA . (1978): Amphetamine-type reinforcement by dopaminergic agonists in the rat. Psychopharmacology 58: 289–296
Acknowledgements
This is publication number 12098-NP from The Scripps Research Institute. This work was supported by NIDA grant DA07348 (FW), and by a fellowship from Singer Polignac and Simone et Cino Del Duca Fondations (RMF). The authors thank Martine Michaud for the synthesis of BTCP, Diana Smith for technical assistance and Mike Arends for assistance with the preparation of the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin-Fardon, R., Weiss, F. N-[1-(2-Benzo[b]Thiophenyl)Cyclohexyl]- Piperidine (BTCP) Exerts Cocaine-Like Actions on Drug-Maintained Responding in Rats. Neuropsychopharmacol 23, 316–325 (2000). https://doi.org/10.1016/S0893-133X(00)00104-4
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(00)00104-4