Abstract
The serotonergic system is involved in the modulation of prepulse inhibition (PPI) and habituation of startle, which are deficient in schizophrenia patients. PPI is the reduction in startle amplitude that occurs when a weak “prepulse” precedes a startling stimulus by 30–500 msec. The roles of 5-HT1A and 5-HT1B receptors in modulating PPI and habituation were examined using wild-type (WT), 5-HT1A knockout (1AKO), and 5-HT1B knockout (1BKO) mice. The 5-HT1A/1B agonist RU24969 reduced PPI and habituation in WT and 1AKO, but not 1BKO mice, whereas the 5-HT1A agonist 8-OH-DPAT increased PPI in WT and 1BKO, but not in 1AKO mice. Similarly, the selective 5-HT1B agonist anpirtoline reduced PPI in WT, but not in 1BKO mice. In experiments using intact 129Sv mice, the 5-HT1A agonist flesinoxan increased PPI while anpirtoline decreased PPI and habituation. Findings suggest that 5-HT1B receptor activation decreases PPI and habituation, and 5-HT1A receptor activation increases PPI in mice.
Similar content being viewed by others
Main
Sensorimotor gating is a form of central nervous system inhibition that “gates” or filters out excessive sensory, motor, and cognitive information to sustain mental and behavioral integration. Prepulse inhibition (PPI), an operational measure of sensorimotor gating, is the reduction in startle amplitude that results when an abrupt startling stimulus is preceded 30–500 msec by a non-startling prepulse (Graham 1975). Patients with several neuropsychiatric disorders, including schizophrenia (Braff et al. 1978, 1992; Bolino et al. 1994), schizotypal personality disorder (Cadenhead et al. 1993), and obsessive compulsive disorder (Swerdlow 1995) exhibit PPI deficits. Startle habituation, a simple form of learning, which refers to the decrement in startle responding to repeated presentations of an initially novel and intense stimulus (Thorpe 1956), is also deficient in schizophrenia and schizotypal patients (Braff et al. 1992; Cadenhead et al. 1993; Geyer and Braff 1982; Bolino et al. 1994). Further investigation of the neural substrates modulating PPI may provide insight into the pathophysiology of the sensorimotor gating deficits in these syndromes.
Serotonin1A (5-HT1A) and serotonin1B (5-HT1B) receptors have been suggested to contribute to the modulation of PPI. For instance, the 5-HT1A agonist 8-OH-DPAT decreases PPI in rats (Rigdon and Weatherspoon 1992; Sipes and Geyer 1995), but increases PPI in mice (Dulawa et al. 1997, 1998). However, 8-OH-DPAT also has affinity for 5-HT7 receptors (Tsou et al. 1994; Ying and Rusak 1997). The 5-HT1A/1B agonist RU24969 decreases PPI and habituation in wild-type (WT), but not 5-HT1B knockout (1BKO) mice (Dulawa et al. 1997), suggesting that 5-HT1B receptors modulate both forms of startle plasticity in mice.
The recent availability of 5-HT1A knockout (1AKO) mice (Ramboz et al. 1998; Heisler et al. 1998; Parks et al. 1998) and the selective 5-HT1B agonist anpirtoline (Schlicker et al. 1992) permits more precise dissection of the roles of 5-HT1A and 5-HT1B receptors in behaviors of interest. 5-HT1A receptors are autoreceptors and heteroreceptors for serotonergic and nonserotonergic neurons and are located on cell bodies and dendrites, where they inhibit cell firing (Hamon et al. 1990). 5-HT1B receptors are autoreceptors and heteroreceptors expressed on axon terminals that inhibit neurotransmitter release (Boschert et al. 1994; Hen 1992). The densest localization of 5-HT1A receptors is in the hippocampus, dorsal raphe, interpeduncular nucleus, and lateral septum (Ramboz et al. 1998). 5-HT1B receptors are most densely localized on striatal neuron terminals in the globus pallidus and substantia nigra, and on Purkinje neuron terminals in the deep cerebellar nuclei (Boschert et al. 1994; Pazos et al. 1988). Many of these brain regions, such as the hippocampus (Caine et al. 1991), septum (Koch 1996), raphe (Sipes and Geyer 1995), and ventral pallidum (Swerdlow et al. 1990, 1992; Sipes and Geyer 1997), are components of the neural circuitry involved in modulating PPI.
We assessed the roles of 5-HT1A and 5-HT1B receptors in modulating PPI and habituation using WT, 1AKO, and 1BKO mice, which are without any obvious anatomical or behavioral abnormalities (Ramboz et al. 1998; Saudou et al. 1994). WT, 1AKO, and 1BKO mice received saline, the 5-HT1A/1B agonist RU24969, which binds preferentially at 5-HT1B sites (Sills et al. 1984; Doods et al. 1985), and the 5-HT1A agonist 8-OH-DPAT. Then, the selective 5-HT1A agonist flesinoxan (Leishman et al. 1994; Schoeffter and Hoyer 1988) was tested in intact 129Sv mice to confirm the PPI-increasing effects of 8-OH-DPAT. Lastly, we assessed whether anpirtoline would disrupt PPI more robustly than RU24969 due to the selectivity of anpirtoline for 5-HT1B and not 5-HT1A receptors (Schlicker et al. 1992). Therefore, a dose response study of anpirtoline was conducted with intact 129Sv mice, and the optimal dose for PPI disruption was then tested in WT and 1BKO mice.
MATERIALS AND METHODS
Subjects
For all experiments, WT and knockout mice were females on a pure 129Sv background (for details, see Phillips et al. 1999) received from the laboratory of Dr. Rene Hen, Columbia University, New York, NY. WT and knockout mice were the offspring of either heterozygote breeders or homozygote breeders no more than three generations removed from a heterozygote cross.
For Experiment 1, ten WT, fifteen 1BKO, and ten 1AKO mice 16–18 weeks old and weighing 15–27 g were the subjects. For Experiment 2, twenty-four female 129SvEms-+Ter?/J mice (Jackson Labs, Bar Harbor, Maine) 10–12 weeks old were the subjects (14–21 g). For Experiment 3, thirty female 129SvEms-+Ter?/J mice (Jackson Labs, Bar Harbor, Maine) 7–9 weeks of age were the subjects (13–20 g). For Experiment 4, ten WT and ten 1BKO female mice 16–18 weeks old were the subjects (17–25 g).
Mice were housed in groups of four with same-type mice in a vivarium with a 12 L : 12 D schedule (lights off at 0900 h). Food and water were available ad libitum. Testing occurred during the dark phase and was in accord with the “Principles of laboratory animal care” NIH guidelines and with local animal care committee approval.
Drugs
5-methoxy-3(1,2,3,6)tetrahydropyridin-4-yl-1H-indole (RU24969) was provided by Roussel UCLAF (Romainville, Cedex, France). The 8-hydroxy-2(di-n-propylamino)-tetralin (8-OH-DPAT) was obtained from Research Biochemicals (Natick, MA). Flesinoxan was provided by Solvay Pharma (Weesp, The Netherlands). The 6-chloro-2-(piperidyl-4-thio)-pyridine (anpirtoline hydrochloride) was purchased from Tocris Cookson (Ballwin, MO). All compounds were made fresh daily and dissolved in 0.9% saline as salt doses, except for flesinoxan, which was dissolved in distilled water.
Injections
Injections were given with 0.5 cc insulin syringes and 28 gauge needles. Solutions were prepared at a volume of 5 ml/kg bodyweight. RU24969 was administered intraperitoneally (i.p.); 8-OH-DPAT, anpirtoline, and flesinoxan were administered subcutaneously (s.c.). Saline or distilled water was administered i.p. or s.c., as appropriate.
Apparatus
Each startle chamber consisted of a nonrestrictive Plexiglas cylinder 5 cm in diameter, resting on a Plexiglas platform in a ventilated chamber (San Diego Instruments, San Diego, CA). A high-frequency speaker mounted 33 cm above the cylinder produced all acoustic stimuli. Mouse movements were detected and transduced by a piezoelectric accelerometer located under the cylinder, and then digitized and stored by a computer and interface assembly. Sixty-five 1-msec readings were recorded to obtain the amplitude of the animals’ startle response to each stimulus, beginning at startling stimulus onset. Sound levels were measured as described elsewhere (Mansbach et al. 1988) using the A weighting scale.
Test Session
Mice were exposed to five different types of discrete stimuli or “trials”: a 40-msec broadband 120 dB burst (Pulse Alone trial); three different Prepulse + Pulse trials in which either 20-msec long 3 dB, 6 dB, or 12 dB above background stimuli preceded the 120 dB pulse by 100 msec (onset to onset); and a No Stimulus trial, in which only the background noise was presented. Trials were presented in a pseudo-random order, separated by an average of 15 s (range: 7–23 s). The test session began with a 5-min acclimation period, followed by four consecutive blocks of test trials. Blocks one and four consisted of six consecutive Pulse Alone trials, while blocks two and three each contained six Pulse Alone trials, five of each kind of Prepulse + Pulse trials, and five No Stimulus trials. Thus, the entire 22-min session consisted of 62 test trials.
Behavioral Testing
Experiment 1
WT, 1BKO, and 1AKO mice were tested in the startle session on three different test days in a within-subjects design, with one rest day separating each test day. Each animal received saline, 10 mg/kg RU24969, and 5 mg/kg 8-OH-DPAT in a counterbalanced order. Although both RU24969 and 8-OH-DPAT have similar effects on PPI at lower doses, the present doses affect PPI more reliably (Dulawa et al. 1997). Animals were placed into testing chambers 30 min after injection.
Experiment 2
Mice received either 0, 0.1, 1, or 10 mg/kg flesinoxan in a between-subjects design. Animals received injections 30 min before startle testing.
Experiment 3
Mice received either 0, 5, or 10 mg/kg anpirtoline in a between-subjects design, 15 min before startle testing.
Experiment 4
WT and 1BKO mice received either saline or 5 mg/kg anpirtoline in a between-subjects design, 15 min before startle testing. The 5 mg/kg dose of anpirtoline was selected based on the results of Experiment 3.
Data Analyses
PPI was calculated as [100 − (prepulse-Pulse trial / Pulse Alone) × 100]. Pulse Alone trials in this calculation were the averaged Pulse Alone values for blocks 2 and 3. Large PPI values indicate substantial inhibition of the startle response by the prepulse, whereas small PPI values indicate little inhibition of the startle response by the prepulse. When an ANOVA assessing PPI yielded no interaction including all factors, data were collapsed for prepulse intensity to reduce the number of post-hoc ANOVAs required for Experiment 1, and to avoid loss of power.
Two different startle reactivity measures were assessed: 1) initial startle reactivity in block 1; and 2) averaged blocks 2 and 3 startle reactivity, which is used for the calculation of PPI. Block 1 startle reactivity was analyzed to evaluate potential phenotypic differences and drug effects on startle reactivity, while avoiding the confounding effects of habituation. Averaged blocks 2 and 3 startle reactivity was assessed to evaluate potential confounds in the calculation of PPI. Startle habituation was assessed as the average startle reactivity to Pulse Alone trials across 4 blocks. In addition, bodyweights of WT, 1AKO, and 1BKO mice were compared using ANOVA.
For Experiment 1, analysis began with four separate ANOVAs for each measure (startle, PPI, habituation). Each ANOVA compared the effects of saline and one drug on WT mice and one kind of knockout mouse: (WT, 1BKO, saline, RU24969); (WT, 1BKO, saline, 8-OH-DPAT); (WT, 1AKO, saline, RU24969); and (WT, 1AKO, saline, 8-OH-DPAT). Alpha was set at p < .05. Significant interactions were examined using post-hoc ANOVAs with alpha levels adjusted for multiple comparisons using the Bonferroni procedure (Winer et al. 1991).
For Experiments 2–4, analysis began with an ANOVA for each measure (startle, PPI). Alpha was set at p < .05. Newman Keuls post-hoc tests were used to compare means when significant interactions (Experiments 1 and 4) or main effects (Experiments 2 and 3) were found for PPI. When significant interactions were found for habituation, post-hoc ANOVAs were applied and alpha levels were adjusted using the Bonferroni procedure.
RESULTS
Experiment 1: 1AKO and 1BKO Mice
Prepulse Inhibition
1BKO mice exhibited significantly higher PPI levels than WT mice (Figure 1a), as revealed by a 2 × 2 ANOVA assessing the effects of saline and 10 mg/kg RU24969 on WT and 1BKO mice and yielding a main effect of genotype [F(1,23) = 8.73, p < .01]. A main effect of prepulse intensity was observed in every analysis for all experiments, confirming the normal relationship in which PPI levels increase with increasing prepulse intensities.
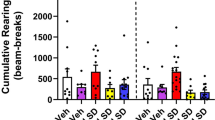
RU24969 decreases PPI in WTs and 1AKOs and 8-OH-DPAT increases PPI in WTs and 1BKOs. (a) PPI is shown collapsed across prepulse intensity for WT (n = 10), 1AKO (n = 19), and 1BKO (n = 15) mice treated with saline, 10 mg/kg RU24969, and 5 mg/kg 8-OH-DPAT. Values are means ± SEM. An asterisk (*) indicates a significant difference from saline within the same genotype. A letter “x” reflects the main effect for RU24969 to decrease PPI across WT and 1AKO mice. A dash (–) reflects the main effect for 1BKO mice to exhibit higher PPI levels than WT mice across treatment with saline and RU24969. (b) Average startle reactivity for blocks 2 and 3 is shown
Treatment with 10 mg/kg of RU24969 decreased PPI across WT and 1AKO mice. This result was confirmed by an ANOVA collapsing for prepulse intensity and yielding a main effect of drug [F(1,18) = 6.07, p < .03].
8-OH-DPAT treatment increased PPI in both WT and 1BKO mice. Analysis yielded a main effect of drug [F(1,23) = 82.84, p < .0001], and an interaction of genotype × drug [F(1,23) = 4.77, p < .04]. Two post-hoc ANOVAs comparing the effects of saline and 8-OH-DPAT in either WT [F(1,9) = 36.81, p < .0005] or 1BKO mice [F(1,14) = 41.77, p < .0001] found a main effect of drug.
8-OH-DPAT treatment increased PPI in WT, but not 1AKO mice. An ANOVA assessing the effects of saline and 5 mg/kg 8-OH-DPAT in WT and 1AKO mice yielded a main effect of drug [F(1,18) = 22.04, p < .0005] and an interaction of genotype × drug [F(1,18) = 20.65, p < .0005]. A post-hoc ANOVA comparing the effects of saline and 8-OH-DPAT in WT mice yielded a main effect of drug [F(1,9) = 36.81, p < .005]. Another post-hoc ANOVA comparing 8-OH-DPAT-treated WT and 1AKO mice revealed a main effect of genotype [F(1,18) = 13.44, p < .005], indicating that 8-OH-DPAT-treated WT mice had higher PPI levels than 8-OH-DPAT-treated 1AKO mice.
Startle Reactivity
8-OH-DPAT treatment significantly decreased averaged blocks 2 and 3 startle reactivity in WT mice (Figure 1b). Additionally, both saline-treated 1AKO and 1BKO mice showed trends for reduced startle reactivity compared to saline-treated WT mice. The ANOVA assessing the effects of saline and 10 mg/kg RU24969 on WT and 1AKO mice yielded a genotype × drug interaction [F(1,18) = 12.63, p < .005]. In addition, the ANOVA assessing the effects of saline and 5 mg/kg 8-OH-DPAT on WT and 1BKO mice yielded a main effect of drug [F(1,23) = 36.5, p < .0001] and a genotype × drug interaction [F(1,23) = 8.82, p < .01]. Likewise, the ANOVA comparing the effects of saline and 8-OH-DPAT on WT and 1AKO mice yielded a main effect of drug [F(1,18) = 38.72, p < .0001] and a genotype × drug interaction [F(1,18) = 31.19, p < .0001]. Two post-hoc ANOVAs confirmed a trend for a main effect of genotype in saline-treated WT and 1BKO mice [F(1,23) = 4.99, p = .04, α = .0125] and saline-treated WT and 1AKO mice [F(1,18) = 4.40, p = .05, α = .0125]. A third post-hoc ANOVA confirmed that 8-OH-DPAT-treatment decreased startle reactivity in WT mice by yielding a main effect of drug [F(1,9) = 55.0 p < .0001]. 1BKO mice (23.0 ± 0.7g) weighed significantly more than both WT (19.2 ± 0.4 g) and 1AKO (20.3 ± 0.9 g) mice, which did not differ from one another. However, these small differences in average weight should not have affected startle values.
Treatment with 8-OH-DPAT and RU24969 decreased block 1 startle reactivity in WT mice, and both saline-treated 1AKO and 1BKO mice showed trends for reduced block 1 startle magnitude relative to WT mice (Figure 2). All four 2 × 2 ANOVAs evaluating the effects of genotype and drug on block 1 startle reactivity found significant main effects of drug, and genotype × drug interactions: the ANOVA assessing the effects of saline and 8-OH-DPAT on WT and 1AKO mice [F(1,18) = 26.60, p < .0001]; the ANOVA assessing the effects of saline and RU24969 on WT and 1AKO mice [F(1,18) = 11.94, p < .005]; the ANOVA assessing the effects of saline and RU24969 on WT and 1BKO mice [F(1,23) = 10.26, p < .005]; and the ANOVA assessing the effects of saline and 8-OH-DPAT on WT and 1BKO mice [F(1,23) = 7.27, p = .0129, (a trend)]. Two post-hoc ANOVAs confirmed with main effects of drug that 8-OH-DPAT [F(1,9) = 29.20, p < .0005] and RU24969 [F(1,9) = 16.64, p < .005] decreased startle reactivity in WT mice. Two additional post-hoc ANOVAs confirmed trends for 1AKO mice [F(1,18) = 3.63, p = .07, α = .0125] and 1BKO mice [F(1,23) = 4.51, p = .04, α = .0125] to exhibit reduced startle magnitude relative to WT mice.
RU24969 decreases habituation in WTs and 1AKOs, but not 1BKOs. Startle reactivity is shown at each block for WT (n = 10), 1AKO (n = 10), and 1BKO (n = 15) mice treated with saline, 10 mg/kg RU24969, and 5 mg/kg 8-OH-DPAT. Values are means ± SEM. An asterisk (*) indicates a significant difference from saline within the same genotype. A letter “x” reflects the main effect for RU24969 to decrease habituation across WT and 1AKO mice. Symbols for block 1 values indicate differences in initial startle reactivity; symbols for block 4 values indicate differences in habituation
Habituation
Habituation was not altered in any group of mice by 5 mg/kg 8-OH-DPAT (Figure 2). The two ANOVAs assessing the effects of saline and 5 mg/kg 8-OH-DPAT in the different genotypes yielded no interactions of genotype or drug with block.
Treatment with 10 mg/kg RU24969 decreased habituation across WT and 1AKO mice. The 2 × 2 × 4 ANOVA assessing the effects of saline and 10 mg/kg RU24969 in WT and 1AKO mice revealed a significant drug × block interaction [F(3,54) = 8.56, p < .0001].
RU24969-treatment decreased habituation in WT, but not 1BKO mice. The ANOVA assessing the effects of saline and 10 mg/kg RU24969 in WT and 1BKO mice revealed a trend for a genotype × drug × block interaction [F(3,69) = 2.46, p = .069]. Post-hoc ANOVAs were based on this trend and the a priori hypothesis derived from previous studies of 5-HT1B receptor influences on habituation (Dulawa et al. 1997). The post-hoc 2 × 4 ANOVA assessing the effects of saline and RU24969 on habituation in WT mice revealed an interaction of drug × block [F(3,27) = 4.82, p < .01], confirming that RU24969 decreased habituation in WT mice. However, the post-hoc ANOVA assessing their effects in 1BKO mice found no drug × block interaction, confirming that RU24969 had no effect on habituation in 1BKO mice. Finally, a post-hoc ANOVA assessing habituation in RU24969-treated WT and 1BKO mice yielded a significant genotype × block interaction [F(3,69) = 5.89, p < .001] confirming that RU24969-treated WT mice exhibited reduced habituation compared to RU24969-treated 1BKO mice.
Experiment 2: Flesinoxan
Prepulse Inhibition
A 3 × 4 ANOVA revealed a main effect of flesinoxan [F(3,20) = 6.08, p < .005], and a drug × prepulse intensity interaction [F(6,40) = 5.99, p < .0005]. Newman Keuls post-hoc tests indicated that 10 mg/kg flesinoxan increased PPI relative to all other doses at the six and 12 dB prepulse intensities (Figure 3).
Startle Reactivity
Neither block 1 nor averaged blocks 2 and 3 startle reactivity was altered by any dose of flesinoxan.
Habituation
The effects of flesinoxan on habituation in intact 129Sv mice were assessed with a 4 × 4 ANOVA. A significant interaction of drug × block was found [F(9,60) = 2.33, p < .03]. However, this interaction resulted from large variations in block two and three values, and not from differences in habituation across all four blocks.
Experiment 3: Anpirtoline
Prepulse Inhibition
A one-way ANOVA collapsing across prepulse intensity revealed a main effect of drug [F(2,27) = 5.90, p < .01]. Newman Keuls post-hoc tests indicated that 5 and 10 mg/kg anpirtoline decreased PPI relative to saline (Figure 4).
Startle Reactivity
Neither block 1 nor averaged blocks 2 and 3 startle reactivity was altered by any dose of anpirtoline.
Habituation
A 3 × 4 ANOVA evaluated the effects of anpirtoline on habituation. A trend for a drug × block interaction was found [F(6,81) = 1.73, p = .12], suggesting that anpirtoline reduces habituation (Figure 5). Post-hoc ANOVAs were based on this trend and the a priori hypothesis that the 5-HT1B receptor influences habituation (Dulawa et al. 1997). Post-hoc ANOVAs revealed trends for 5 mg/kg [F(3,54) = 1.97, p = .13] and 10 mg/kg [F(3,54) = 1.96, p = .13] anpirtoline to decrease habituation relative to saline.
Experiment 4: 1BKO Mice and Anpirtoline
Prepulse Inhibition
A 2 × 2 ANOVA collapsing across prepulse intensity revealed a main effect of genotype [F(1,16) = 6.69, p < .02] and a trend for a genotype × drug interaction [F(1,16) = 3.32, p = .087]. Newman Keuls post-hoc tests indicated that WT mice treated with 5 mg/kg anpirtoline had lower PPI levels than all other groups of mice. Thus, anpirtoline disrupted PPI in WT but not 1BKO mice (Figure 6).
Anpirtoline decreases PPI in WT, but not 1BKOs. PPI is shown collapsed across prepulse intensity for WT (n = 10) and 1BKO (n = 10) mice receiving 0 or 5 mg/kg anpirtoline. Values are means ± SEM. An asterisk (*) indicates a significant difference from saline within the same genotype. A pound sign (#) indicates a significant difference from WT mice within the same treatment group
Startle Reactivity
A trend for a main effect of genotype for averaged blocks 2 and 3 startle reactivity [F(1,16) = 4.02, p = .06] was indicated by ANOVA. Newman Keuls post-hoc tests did not find any differences between means. 1BKO mice (23.4 ± 0.7 g) weighed significantly more than WT mice (20.3 ± 0.9 g), however this average difference of 3.1 g should not have affected startle values.
ANOVA also indicated a trend for a main effect of genotype for block 1 startle reactivity [F(1,16) = 3.66, p = .07]. No differences between genotypes were indicated by Newman Keuls post-hoc tests.
Habituation
The 2 × 2 × 4 ANOVA assessing the effects of saline and 5 mg/kg anpirtoline in WT and 1BKO mice found no interactions of genotype or drug with block. Thus, neither genotype nor anpirtoline had effects on habituation.
DISCUSSION
The present results indicate that the activation of 5-HT1A receptors increases PPI, whereas the activation of 5-HT1B receptors decreases both PPI and habituation in mice. The 5-HT1A agonist 8-OH-DPAT increased PPI in WT and 1BKO, but not 1AKO mice, whereas the 5-HT1A /1B agonist RU24969 decreased both PPI and habituation in WT and 1AKO, but not 1BKO mice. Furthermore, the 5-HT1B agonist anpirtoline decreased PPI in WT, but not 1BKO mice. Consistent with these results, the 5-HT1A agonist flesinoxan increased PPI, whereas anpirtoline decreased both PPI and habituation in intact 129Sv mice. The opposite roles of 5-HT1A and 5-HT1B receptors in modulating PPI are consistent with their opposite effects on a number of other behavioral responses (Zhuang et al. 1999).
No phenotypic difference in PPI was observed in 1AKO mice, although a small increase in PPI was found in 1BKO mice (Figure 1). The phenotypic increase in PPI observed in 1BKO mice is consistent with earlier reports (Dulawa et al. 1997) and the present findings that RU24969 and anpirtoline decrease PPI in WT, but not 1BKO mice. The small phenotypic increase in PPI may result from lack of the normal activation of 5-HT1B receptors by endogenous serotonin. 1AKO mice did not show a phenotypic decrease in PPI, which might have been expected, because 8-OH-DPAT and flesinoxan increase PPI. Hence, under non-challenged conditions, the endogenous serotonergic tone may be insufficient to modulate PPI via 5-HT1A receptors, although sufficient to do so via 5-HT1B receptors. Alternatively, developmental compensations specific to the 1AKO mice may obscure the effects of absence of the receptor on PPI.
In Experiment 1, a total of 5 mg/kg of 8-OH-DPAT increased PPI in WT and 1BKO, but not 1AKO mice. The reduction in startle reactivity produced by 8-OH-DPAT in WT mice is unlikely to be responsible for the increase in PPI, because 8-OH-DPAT increased PPI in 1BKO mice without significantly altering startle reactivity (Figure 1). Furthermore, 10 mg/kg of the selective 5-HT1A agonist flesinoxan increased PPI without altering startle reactivity (Figure 3). The PPI-increasing effects of 8-OH-DPAT and flesinoxan in mice are intriguing because 8-OH-DPAT and other 5-HT1A agonists decrease PPI in rats (Rigdon and Weatherspoon 1992; Sipes and Geyer 1995). We previously observed that 8-OH-DPAT increases PPI in mice, and that this effect could be prevented by pretreatment with the 5-HT1A antagonist WAY100,635 (Dulawa et al. 1998). However, 8-OH-DPAT and a number of 5-HT1A antagonists have some affinity for the 5-HT7 receptor (Tsou et al. 1994; Ying and Rusak 1997). Thus, the lack of effect of 8-OH-DPAT on PPI in 1AKO mice provides evidence that the effects of 8-OH-DPAT on PPI are mediated by 5-HT1A receptors and not 5-HT7 receptors.
Treatment with 10 mg/kg RU24969 reduced PPI across WT and 1AKO, but not 1BKO mice. However, RU24969 appeared to decrease PPI more robustly in 1AKO mice than in WT (Figure 1). This apparent difference may have resulted from the PPI-increasing effect of RU24969 at 5-HT1A receptors in WT mice. Consistent with this idea is the finding that the selective 5-HT1B agonist anpirtoline produced a robust PPI disruption in WT, but not 1BKO mice (Figure 6). Thus, the present results indicate that the activation of 5-HT1B receptors disrupts PPI more robustly than previously suggested.
Constitutive genetic manipulations can lead to compensatory changes that may influence the behavioral phenotype. Scearce et al. (1997) reported a 15% increase in dopamine transporter binding in the dorsolateral striatum, a 20% increase in dopamine D1 receptor binding in the anterior ventrolateral striatum, a 20% decrease in serotonin transporter binding, and an increase in D1 receptor mRNA in whole striatum of 1BKO mice. Although autoradiographic analysis has not revealed changes in 5-HT1A receptor binding in 1BKO mice (Hen, unpublished observations), the present findings might reflect some functional desensitization of 5-HT1A receptors in 1BKO mice. For example, 8-OH-DPAT appeared to produce a less robust increase in PPI in 1BKO mice than in WT mice (Figure 1), which has been observed consistently (Dulawa et al. 1997). Although this result may represent a ceiling effect, there is also an absence of a 1A-effect (increased PPI) in 1BKO mice treated with RU24969. One might have expected to find a small increase in PPI due to the moderate affinity of RU24969 for the 5-HT1A receptor; the 1A-effect can be observed when comparing the effect of RU24969 on PPI in WT and 1AKO mice. Some compensatory changes have also been reported in 1AKO mice. For example, 5-HT1B receptors may be upregulated (Ramboz et al. 1998), although there is little evidence for a behavioral consequence of this upregulation in the present experiments.
The activation of 5-HT1A receptors increases PPI in mice (Dulawa et al. 1997, 1998), and decreases PPI in rats (Sipes and Geyer 1995; Rigdon and Weatherspoon 1992). Inconsistencies have also been reported regarding the effects of drugs on PPI in rats versus humans. For example, the serotonin releaser 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) decreases PPI in rats (Kehne et al. 1992; Mansbach et al. 1989; Vollenweider et al. 1999), but increases PPI in healthy humans (Vollenweider et al. 1999). In addition, MDMA decreases PPI in some substrains of 129 mice (Dulawa and Geyer 1996), but not in others (Dulawa et al. 1998). These conflicting results raise the question as to which rodent model will more closely resemble humans. Flesinoxan is currently being investigated as a potential antidepressant in humans (Baumann and Bertschy 1997; Grof et al. 1993). Thus, assessment of the effects of flesinoxan on PPI in humans might soon be possible. Because 5-HT1A receptors have a similar distribution pattern in the mouse (Ramboz et al. 1998), the rat (Marcinkiewicz et al. 1984; Pompeiano et al. 1992), and humans (Pazos et al. 1987; Hoyer et al. 1986), unique distribution patterns are an unlikely reason for the species-specific effects of 8-OH-DPAT on PPI. A more plausible explanation is a difference in the balance of pre- and postsynaptic receptor signaling between species. Study of these differences could greatly improve our understanding of the neural circuits modulating these behaviors, and allow the selection of the most appropriate animal model for addressing specific hypotheses.
Trends were found for initial startle reactivity to be decreased in 1AKO and 1BKO mice relative to WT, although there were no significant differences between groups (Figure 2). We previously observed a significant decrease in block 1 startle reactivity in 1BKO male mice, and no effect on block 1 startle reactivity in 1BKO female mice, all relative to WT mice (Dulawa et al. 1997). 1AKO mice have been reported to have increased anxiety (Ramboz et al. 1998; Parks et al. 1998; Heisler et al. 1998), whereas 1BKO mice have somewhat decreased anxiety (Ramboz et al. 1998; Brunner and Hen 1997) in the same behavioral tests. Yet, a trend for decreased initial startle reactivity was observed in both 1AKO and 1BKO mice compared to WT mice. This discrepancy suggests that baseline startle reactivity may not be a sensitive measure of anxiety, or alternatively, that different neural circuits mediate different aspects of anxiety.
Startle habituation phenotypes were comparable in WT, 1AKO, and 1BKO mice. However, 10 mg/kg RU24969 disrupted habituation in WT and 1AKO, but not 1BKO mice (Figure 2), consistent with an earlier report (Dulawa et al. 1997) assessing WT and 1BKO mice. This finding indicates that activation of 5-HT1B receptors decreases habituation, and that 5-HT1A receptors are not involved in the RU24969-induced decrease in habituation. This disruption of habituation was not confounded by significant differences in block 1 startle reactivity between RU24969-treated WT, 1AKO, or 1BKO mice. A trend for anpirtoline to disrupt habituation was also observed in intact 129Sv mice (Figure 5). Although anpirtoline did not alter habituation in WT mice in Experiment 4, this discrepancy was probably due to the fact that three times as many intact mice were used in Experiment 3 (30 129Sv mice) as in Experiment 4 (10 WT), and that startle responses show considerable variability. No changes in habituation resulted from flesinoxan or 8-OH-DPAT treatment in any group of mice, as reported with rats (Geyer and Tapson 1988). The lack of effect of 8-OH-DPAT in 1AKO mice suggests that 5-HT7 receptors do not modulate habituation. Together, these findings indicate that 5-HT1B but not 5-HT1A receptors modulate habituation in mice.
Lack of the 5-HT1A or 5-HT1B receptor does not induce substantial changes in the expression of PPI or habituation. However, under drug-challenged conditions, 5-HT1B receptor activation decreases PPI and habituation, and 5-HT1A receptor activation increases PPI. Because 5-HT1A and 5-HT1B receptors are important for the modulation of sensorimotor gating, future experiments using tissue-specific knockout manipulations (Stark et al. 1998) to examine their pre- and postsynaptic contributions to these behaviors are warranted.
References
Baumann P, Bertschy G . (1997): Long-term treatment of depression: Is there a use for depot antidepressants? Intl Clin Psychopharmacol 12: 77–80
Bolino F, Di Michele V, Di Cicco L, Manna V, Daneluzzo E, Casacchia M . (1994): Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenia. Biol Psychiatry 36: 670–679
Boschert U, Amara DA, Segu L, Hen R . (1994): The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience 58: 167–182
Braff DL, Stone C, Callaway E, Geyer MA, Glick I, Bali L . (1978): Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology 15: 339–343
Braff DL, Grillon C, Geyer MA . (1992): Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 49: 206–215
Brunner D, Hen R . (1997): Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. In Stoff DM, Mann JJ (eds), The Neurobiology of Suicide: From the Bench to the Clinic. New York, New York Academy of Sciences, pp 81–105
Cadenhead KS, Geyer MA, Braff DL . (1993): Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry 180: 1862–1870
Caine SB, Geyer MA, Swerdlow NR . (1991): Carbachol infusion into the dentate gyrus disrupts sensorimotor gating of startle in the rat. Psychopharmacology 105: 347–354
Doods HN, Kalkman HO, De Jinge A, Thoolen MJMC, Wilffert B, Timmermans PBMWM, Van Zwieten PA . (1985): Differential selectivities of RU 24969 and 8-OH-DPAT for the purported 5-HT1A and 5-HT1B binding sites. Correlation between 5-HT1A affinity and hypotensive activity. Eur J Pharm 112: 363–370
Dulawa SC, Geyer MA . (1996): Psychopharmacology of prepulse inhibition in mice. Chinese J Physiol 39: 1–8
Dulawa SC, Hen R, Scearce-Levie K, Geyer MA . (1997): Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild type and serotonin1B knockout mice. Psychopharmacology 132: 125–134
Dulawa SC, Hen R, Scearce-Levie K, Geyer MA . (1998): Serotonin1B receptor modulation of prepulse inhibition: Recent findings in wild type and 5-HT1B knockout mice. Ann NY Acad Sci 861: 79–84
Geyer MA, Tapson GS . (1988): Habituation of tactile startle is altered by drugs acting on serotonin-2 receptors. Neuropsychopharmacology 1: 135–147
Geyer MA, Braff DL . (1982): Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology 19: 1–6
Graham FK . (1975): The more or less startling effects of weak prestimuli. Psychophysiology 12: 238–248
Grof P, Joffe R, Kennedy S, Persad E, Syrotiuk J, Bradford D . (1993): An open study of oral flesinoxan, a 5-HT1A receptor agonist, in treatment-resistant depression. Int Clin Psychopharm 8: 167–172
Hamon M, Lanfumey L, El Mestikawy S, Boni C, Miquel MC, Bolanos F, Schechter L, Gozalan H . (1990): The main features of central 5-HT1 receptors. Neuropsychopharmacology 3: 349–360
Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH . (1998): Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA 95: 15049–15054
Hen R . (1992): Of mice and flies: Commonalities among 5-HT receptors. Trends Pharmacol Sci 13: 160–165
Hoyer D, Pazos A, Probst A, Palacios JM . (1986): Serotonin receptors in the human brain. I. Characterization and autoradiographic localization of 5-HT1A recognition sites. Apparent absence of 5-HT1B recognition sites. Brain Res 376: 85–96
Kehne JH, McCloskey TC, Taylor VL, Black CK, Fadayel GM, Schmidt CJ . (1992): Effects of the serotonin releasers 3,4-methylenedioxymethamphetamine (MDMA), 4-chloroamphetamine (PCA) and fenfluramine on acoustic and tactile startle reflexes in rats. J Pharmacol Exp Ther 260: 78–89
Koch M . (1996): The septohippocampal system is involved in prepulse inhibition of the acoustic startle response in rats. Behav Neurosci 110: 468–477
Leishman DJ, Boeijinga PH, Galvan M . (1994): Differential effects of centrally-active antihypertensives on 5-HT1A receptors in rat dorso-lateral septum, rat hippocampus and guinea-pig hippocampus. Br J Pharmacol 111: 318–324
Mansbach RS, Geyer MA, Braff DL . (1988): Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacol 94: 507–514
Mansbach RS, Braff DL, Geyer MA . (1989): Prepulse inhibition of the acoustic startle response is disrupted by N-ethyl-3,4-methylenedioxyamphetamine (MDEA) in the rat. Eur J Pharmacol 167: 49–55
Marcinkiewicz M, Verge D, Gozlan H, Pichat L, Hamon M . (1984): Autoradiographic evidence for the heterogeneity of 5-HT1 sites in the rat brain. Brain Res 291: 159–163
Parks CL, Robinson PS, Sibille E, Shenk T, Toth M . (1998): Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA 95: 10734–10739
Pazos A, Hoyer D, Dietl MM, Palacios JM . (1988): Autoradiography of serotonin receptors. In Osborne NN, Hamon M (eds), Neuronal Serotonin. New York, Wiley, pp 507–543
Pazos A, Probst A, Palacios JM . (1987): Serotonin receptors in the human brain. IV. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 21: 97–122
Phillips TJ, Hen R, Crabbe JC . (1999): Complications associated with genetic background effects in research using knockout mice. Psychopharmacology 147: 5–7
Pompeiano M, Palacios JM, Mengod G . (1992): Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: Correlation with receptor binding. J Neurosci 12: 440–453
Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R . (1998): Serotonin receptor 1A knockout: An animal model of anxiety-related disorder. Proc Natl Acad Sci USA 95: 14476–14481
Rigdon GC, Weatherspoon J . (1992): 5HT1A receptor agonists block prepulse inhibition of the acoustic startle reflex. J Pharmacol Exp Ther 263: 486–493
Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R . (1994): Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265: 1875–1878
Scearce K, Kassir S, Lucas J, Castanon N, Segu L, Arango V, Hen R . (1997): Dopaminergic compensations in knockout mice lacking the serotonin 1B receptor. Abstr Soc Neurosci 23: 202.2
Schlicker E, Werner U, Hamon M, Nickel B, Szelenyi I, Gothert M . (1992): Anpirtoline, a novel, highly potent 5-HT1B receptor agonist with antinociceptive/antidepressant-like actions in rodents. Br J Pharmacol 105: 732–738
Schoeffter P, Hoyer D . (1988): Centrally acting hypotensive agents with affinity for 5-HT1A binding sites inhibit forskolin-stimulated adenylate cyclase activity in calf hippocampus. Br J Pharmacol 95: 975–985
Sills MA, Wolfe BB, Frazer A . (1984): Determination of selective and nonselective compounds for the 5-HT1A and 5-HT1B receptor subtypes in rat frontal cortex. J Pharmacol Exp Ther 231: 480–487
Sipes TA, Geyer MA . (1995): 8-OH-DPAT disruption of prepulse inhibition in rats: Reversal with (+)WAY 100,135 and localization of site of action. Psychopharmacology 117: 41–48
Sipes TE, Geyer MA . (1997): DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res 761: 97–104
Stark KL, Oosting R, Hen R . (1998): Novel strategies to probe the functions of serotonin receptors. Biol Psychiatry 44: 163–168
Swerdlow NR . (1995): Serotonin, obsessive compulsive disorder and the basal ganglia. Int Rev Psychiatry 7: 115–129
Swerdlow NR, Braff DL, Geyer MA . (1990): GABAergic projection from nucleus accumbens to ventral pallidum mediates dopamine-induced sensorimotor gating deficits of acoustic startle in rats. Brain Res 532: 146–150
Swerdlow NR, Caine SB, Braff DL, Geyer MA . (1992): Neural substrates of sensorimotor gating of the startle reflex: Preclinical findings and their implications. J Psychopharmacol 6: 176–190
Thorpe WH . (1956): Learning and Instinct in Animals. Cambridge MA, Harvard University Press
Tsou AP, Kosaka A, Bach C, Zuppan P, Yee C, Tom L, Alvarez R, Ramsey S, Bonhaus DW, Stefanich E, Jakeman L, Eglen RM, Chan HW . (1994): Cloning and expression of a 5-hydroxytryptamine7 receptor positively coupled to adenylyl cyclase. J Neurochem 63: 456–464
Vollenweider FX, Remensberger S, Hell D, Geyer MA . (1999): Opposite effects of 3,4-methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology 143: 365–372
Winer BJ, Brown DR, Michels KM . (1991): Statistical Principles in Experimental Design. New York, McGraw-Hill, Inc
Ying SW, Rusak B . (1997): 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmatic nucleus neurons. Brain Res 755: 246–254
Zhuang X, Gross C, Santarelli L, Compan V, Trillat A-C, Hen R . (1999): Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology 21: 52S–60S
Acknowledgements
The authors acknowledge the generous gifts of flesinoxan from Solvay Pharma, and RU24969 from Roussel UCLAF. This work was supported by grants from the National Institute on Drug Abuse (R02DA02925 & R01DA09862–01), the National Institute of Mental Health (NIMH) P01MH48126–06, the National Alliance for Research on Schizophrenia and Depression (NARSAD), and the U.S. Veterans Affairs VISN22 Mental Illness Research, Education, and Clinical Center. S.C. Dulawa was supported by a predoctoral NRSA from NIMH (F31-MH12249–01). C. Gross was supported by a grant from NIMH (F32MH12364–01). M.A. Geyer holds equity interest in San Diego Instruments, Inc.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dulawa, S., Gross, C., Stark, K. et al. Knockout Mice Reveal Opposite Roles for Serotonin 1A and 1B Receptors in Prepulse Inhibition. Neuropsychopharmacol 22, 650–659 (2000). https://doi.org/10.1016/S0893-133X(99)00164-5
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(99)00164-5
Keywords
This article is cited by
-
Sex-specific effects of psychedelics on prepulse inhibition of startle in 129S6/SvEv mice
Psychopharmacology (2022)
-
Serotonin 5-HT1B receptor-mediated behavior and binding in mice with the overactive and dysregulated serotonin transporter Ala56 variant
Psychopharmacology (2021)
-
Effects of combined 5-HT2A and cannabinoid receptor modulation on a schizophrenia-related prepulse inhibition deficit in mice
Psychopharmacology (2020)
-
Effects of chronic fluoxetine treatment on serotonin 1B receptor-induced deficits in delayed alternation
Psychopharmacology (2013)
-
Serotonin1A receptor deletion does not interact with maternal separation-induced increases in startle reactivity and prepulse inhibition deficits
Psychopharmacology (2011)