Abstract
Native Americans have some of the highest rates of alcohol abuse and dependence, yet potential biological risk factors associated with the problem drinking seen in some tribes remain relatively unknown. The amplitude of the P3 component of the event-related potential (ERP) is perhaps the most studied electrophysiological “marker” of potential vulnerability to alcohol dependence, yet it has not been investigated in Native Americans. Forty-seven, non-alcohol-dependent Native American Mission Indian men between the ages of 18 and 25 years participated in the study. ERPs were collected at 60 minutes following both alcohol (0.56 g/kg) and placebo intake. No relationship was found between P3 amplitude and degree of Native-American heritage (NAH), or family history (FH) of alcohol dependence. The results of this study did, however, replicate previous findings that the P3 component of the ERP is sensitive to the effects of alcohol. A reduction in the P3a component across the scalp was found in these Native American men following alcohol when compared with placebo ingestion. P3 response to alcohol, although not influenced by a subject's NAH or FH, was influenced by the presence of a polymorphism in the alcohol metabolizing enzyme alcohol dehydrogenase (ADH). Men with an ADH2*3 allele had significantly higher amplitude P3 components at placebo and also demonstrated more alcohol-induced reductions in P3 amplitude than men with ADH2*1 alleles only. In addition, individuals with low P3 amplitude in the placebo condition had less of a reduction or an actual increase in P3a and P3b amplitudes following alcohol intake. Given that a less intense response to alcohol has been associated with greater risk for the development of alcohol-related problems, these data suggest the presence of certain biological variables within this Native American population that may confer both risk and protection for the future development of alcohol dependence.
Similar content being viewed by others
Main
The prevalence of alcohol abuse and alcohol dependence among different ethnic groups varies widely. For example, Americans of Jewish descent (Chavetz 1964; Cahalan 1970; Klatsky et al. 1983; Monteiro and Schuckit 1989; Helzer et al. 1990) and various Asian groups (Lin 1953; Singer 1972) have lower than average rates of alcohol dependence. Ethnic groups with a high incidence of alcohol use and alcoholism include Hawaiians, Samoans (Lemert 1964), Irish (Knupfler and Room 1967), and certain tribes of Native Americans (Kunitz et al. 1971; Brod 1975).
Although tribes differ with regard to the use of alcohol, the U.S. Indian Health Service has cited alcoholism as the most urgent health problem facing Native Americans (U.S. Indian Health Service 1977, 1982). Epidemiological studies demonstrate that Native Americans as a whole have: an alcohol dependence rate twice the U.S. national average, a 3-times increased hospital discharge rate for alcohol-related diagnoses, 4.4 times the national mortality rate for cirrhosis of the liver, and a motor vehicle death rate 4.4 times higher than the national rate, which is 50 to 60% alcohol related (see Andre 1979; May 1982; U.S. Indian Health Service 1982). A full 75% of all Native American deaths are related to alcohol in some way, with five of the 10 leading causes of death being directly related to alcohol. Although it is difficult to disentangle genetic from environmental factors in population studies, recent data suggest that Native Americans have the highest prevalence of a positive family history for alcoholism among all ethnic groups. A full 62.8% of women and 46.1% of men reported a family history of alcoholism (Harford 1992).
Findings on the importance of genetic factors in the etiology of alcohol dependence have stimulated a number of investigators to search for biological markers that might mediate increased risk for this disorder. One approach has focused on biological children of alcoholics who are identified at various ages, evaluated, and, in some studies, challenged with alcohol. Most investigations have studied subjects with a family history of alcoholism (FHP), usually defined through alcohol dependence in a biological father. These subjects are compared to control subjects without a family history of alcoholism (FHN) among biological relatives. Many of these types of investigations have studied Caucasian males in their late teens and early twenties (Schuckit 1985). Other studies have focused on children in their prepubertal to early adolescent years (Begleiter et al. 1984; Hill et al. 1990). Individual sensitivity to alcohol has been proposed as an inherited factor that affects the likelihood of drinking and mediates disposition for developing alcoholism (Schuckit 1994). Although not all studies agree (Newlin and Thomson 1990), a meta-analysis focusing on subjective levels of intoxication confirmed a diminished response to alcohol as a characteristic more frequently seen in FHP compared with FHN subjects (Pollock 1992). In addition, a subsequent 8-year follow-up of previously studied FHP and FHN men, found that both a family history of alcoholism and a low response to alcohol were related to the future development of alcohol-related problems (Schuckit and Smith 1996).
Several studies provide evidence to suggest that certain electrophysiological variables may represent “markers” of vulnerability to alcohol dependence. One measure that has received considerable attention as a possible neurophysiological marker for alcoholism risk is the P3 component of the event-related potential (ERP). Many studies provide evidence demonstrating that the amplitude of the P3 is reduced in FHP subjects (see Elmasian et al. 1982; Begleiter et al. 1984; O'Connor et al. 1986; Whipple et al. 1988; Hill et al. 1988, 1990; Porjesz and Begleiter 1990; Berman et al. 1993; Hill and Steinhauer 1993; Steinhauer and Hill 1993; Ramachandran et al. 1996). Other studies are less convincing or do not support the hypothesis (see Polich and Bloom 1987; Polich 1988). A recent meta-analysis, however, has concluded that P3 “may be useful as an index for predicting alcoholism vulnerability” (Polich et al. 1994).
This report is part of a larger study exploring risk factors for alcoholism among non-alcohol-dependent Native American Mission Indian men. The present investigation was specifically designed to explore the relationships between P3 amplitude and factors associated with risk or protection from alcohol dependence. The study evaluated several hypotheses. First we sought to determine whether P3 amplitude was related to family history of alcoholism or percent Native American heritage (NAH) in these Native American men. Second, because P3 response to alcohol has never been reported in Native Americans, we sought to determine if alcohol produced effects similar to those reported in Caucasian and Asian subjects. Third, we investigated the relationship between P3 and two alcohol dehydrogenase genes (ADH2 and ADH3) that are polymorphic in this population (Wall et al. 1997). These candidate genes encode isoenzymes that differ in their kinetic properties (Bosron et al. 1993). A number of studies have examined ADH2 and ADH3 genotype variations to explain susceptibility to alcoholism (Thomasson et al. 1991; Gilder et al. 1993; Higuchi et al. 1995) and other alcohol-related disease in various ethnic groups (Couzigou et al. 1990; Day et al. 1991; Chao et al. 1994; McGarver-May et al. 1996). Finally, since P3 amplitude and response to alcohol have both been demonstrated to be associated to risk for alcoholism, we also sought to determine if lowered P3 amplitude itself was predictive of a less intense response to alcohol challenge in this population.
METHODS
Subjects
A group of Native Americans known collectively as Mission Indians was targeted for study. Individual subjects were recruited from six geographically contiguous Indian reservations in southern California. Males between the ages of 18 and 25 years who were at least 25% NAH according to the federal Indian blood-quantum responded to fliers posted at the Indian Health Clinic, tribal halls, local schools, and on-site stores and were also recruited by word of mouth. Seventy-eight potential subjects were screened by telephone interview. Eighteen subjects were found not eligible for the study by initial phone interview. Of the 60 remaining subjects, 55 agreed to meet individually with research staff to complete a screening questionnaire (Schuckit 1984; Schuckit and Gold 1988) that was used to select individuals who met eligibility for the study. Using this highly structured self-report instrument, subjects were excluded from further evaluation if they met diagnostic criteria for alcohol or other substance dependence, or other major Axis I psychiatric disorders according to criteria outlined in the Third Diagnostic and Statistical Manual Disorders (DSM-III) (American Psychiatric Association 1980). The screening questionnaire was also used to gather information on demography, personal medical history, usual quantity and frequency of alcohol and other drug consumption over the previous 6 months, and family history of alcohol and other substance dependence. Individuals were also excluded from this study if they were taking prescribed medication, had any major medical condition, or had abstained from alcohol over the previous 6 months.
For secondary analyses, a subject was classified as FHP if he had a first-degree relative who met DSM-III criteria for alcohol dependence. Family history positive subjects had a mean of 4.0 ± 2.9 first and second-degree family members with alcohol dependence. Those subjects with a biological mother who met criteria for alcohol dependence during pregnancy were excluded from the study. FHN men lacked a history of alcohol dependence in their first- or second-degree relatives. The subjects were also categorized according to those with greater than or equal to 50% NAH and those with 25–50% NAH by their federal Indian blood quantum. Subjects were screened for the presence of ADH2 and ADH3 polymorphisms using polymerase chain reaction and allele-specific oligonucleotide primers followed by hybridization with radio-labeled oligonucleotide probes (Xu et al. 1988) as previously described (Wall et al. 1997).
Alcohol Challenge
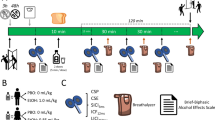
Men (n = 47) who met inclusion criteria were invited to participate individually in two test sessions, approximately 1 week apart, that consisted of baseline evaluations and subsequent challenges with placebo and alcohol. Subjects were instructed not to use alcohol or any other drugs for 3 days prior to testing. On both test days, each man arrived to the laboratory at approximately 8:00 A.M. after fasting overnight and was provided a standardized low-fat breakfast. Baseline measurements were taken and at about 9:00 A.M., a placebo or alcohol beverage was administered in random order, using a placebo alcohol administration device (Mendelson et al. 1984). The alcohol beverage was 0.75 ml/kg of 95% alcohol (0.56 g/kg) as a 20%-by-volume solution in a caffeine-free and sugar-free soda. The placebo beverage was made using the same mixer with 3 ml of 95% alcohol floated on top. Subjects were instructed to drink at a steady pace and to consume the beverage over 7 min. ERPs were collected at 60 min after beverage intake. Electroencephalogram (EEG) was collected before and at 30, 60, and 90 minutes after beverage intake. Heart rate, blood pressure, subjective ratings of alcohol's effects, and blood samples for subsequent determination of blood alcohol concentrations (BACs) and plasma cortisol levels were also obtained before and 15, 30, 60, 90, and 120 minutes after beverage intake. Results from non-ERP measures have been previously reported (Wall et al. 1996, 1997; Garcia-Andrade et al. 1996, 1997; Ehlers et al. 1997).
ERP Collection and Analyses
Three channels of ERP data (FZ, CZ, PZ, and referenced to linked ear lobes with a forehead ground, international 10–20 system) were obtained by using gold-plated electrodes with impedance held below 5K ohms. An electrode placed left lateral infraorbitally and referenced to the left earlobe was used to monitor both horizontal and vertical eye movement. ERP recording signals were amplified (time constant 0.1 s, 35 Hz low pass) and were transferred on-line to a Macintosh computer for digitization. Auditory stimuli were presented through headphones and ERPs were elicited using an oddball plus “noise” paradigm. The acoustic parameters were three square wave tones (rise/fall times <1 ms): a standard tone (50 ms, 2 KHz, 70 dB SPL) presented on 83% of the trials (n = 200), a rare tone (50 ms, 2 KHz, 80 dB SPL) presented on 10.4% of the trials (n = 25), and a noise burst (50 ms, noise, 80 dB spl) presented on 6.3% of the trials (n = 15). Each subject was instructed to depress a counter each time he detected a rare tone and to withhold responding to the standard tones and noise bursts. The tones were generated by a programmable multiple-tone generator, the characteristics of which have been described previously (Polich et al. 1983). Each subject was hearing tested prior to the recording to insure that he had no trouble easily identifying the three different tones. Rare tones were interspersed with standard tones so that no two rare tones occurred consecutively. A noise burst was substituted for a rare tone every 12 trials to avoid habituation to the noise burst. The digitizing epoch was 1 s and a 0.5- to 1.0-s intertrial interval was used. The total number of trials in each recording session was 240. These stimulus characteristics have been used extensively in our laboratory (see Wall and Ehlers 1995; Ehlers et al. 1996).
The ERP trials were digitized at a rate of 256 Hz. Individual trials containing excessive eye movement artifact as well as trials where the EEG exceeded ±250 microvolts (<5% of the trials) were eliminated before averaging. The P3 was defined as the occurrence of a set of peak amplitudes after the N1-P2-N2 complex within 250–400 ms after stimulus presentation. The baseline was determined by averaging the 100 ms of prestimulus activity obtained for each trial. The latency (in ms) was defined as the time of the peak amplitude generally within these latency windows: P3a, 225–300 ms; P3b, 300–400 ms. The P3a and P3b components were quantified using a computerized peak detection routine that identifies baseline-to-peak amplitudes (in μV) within specified latency windows. The routine is user driven, and each peak detection must be verified by the user. All peaks were quantified by one individual (E.P., R EEG Tech), and verified by a second individual (C.E.), both of whom were blind to subject conditions.
Data Analyses
Data analyses focused on three hypotheses that were generated based on previous research. The first question was an analysis of potential determinants of P3 amplitude during the placebo session. Factors explored were: ADH2 genotype, (ADH2*1/2*3, ADH2*1/2*1), ADH3 genotype (ADH3*1/3*1, ADH3*1/3*2, ADH3*2/3*2) family history of alcohol dependence (FHP, FHN) and NAH (>50% NAH, <50% NAH). Multivariate analysis of variance (MANOVA) was utilized to explore the relationship between these hypothesized risk factors and P3a and P3b amplitude at each electrode site. To explore for potential confounding variables between the hypothesized risk groups (NAH, FH, ADH2 genotype, ADH3 genotype), demographic and drinking history variables were analyzed using one-way analysis of variance (ANOVA).
The second question focused on whether significant effects of alcohol on P3 amplitude could be detected in this group of Native American Mission Indians. To address this question, MANOVA was conducted with P3a and P3b amplitude as dependent variables and drug condition (alcohol, placebo) and electrode site (FZ, CZ, PZ) as repeated measurements.
The third hypothesized question focused on whether differences in response to alcohol were modified by the predicted risk/protection factors. In these exploratory analyses FH, NAH, ADH2 genotype, ADH3 genotype, and low voltage P3 group designation, were treated as between-subjects variables and drug (alcohol, placebo) was treated as a repeated measure for the three electrode sites. BACs for the alcohol session were analyzed using two-factor (group × time) ANOVA with group (FH, NAH, or ADH2, ADH3, low voltage P3) as a between-subjects variable and time as a repeated measurement.
RESULTS
Fifty-five subjects met inclusion/exclusion criteria by telephone screening and were subsequently interviewed in person. Of these, 51 men met inclusion and exclusion criteria by interview (two were eliminated for a history of psychosis, one had a neurologic disorder, and one met criteria for drug dependence). Three subjects did not complete the placebo session, and one fell asleep during the recording of ERPs. The 47 remaining subjects had a mean ± SD age of 21.2 ± 2.3. They drank an average of 4.4 ± 3.3 days per month, and consumed an average of 5.0 ± 3.0 drinks per occasion. Demographic and substance use history are presented in Table 1.
To address the first major research question, P3 amplitude recorded during the placebo session was evaluated, as a function of the hypothesized risk factors within this Native American population. The four risk factors FHP vs. FHN, <50% NAH vs. ≥50% NAH, ADH2*1/2*3 vs. ADH2*1/2*1, ADH3*1/3*1 vs. ADH3*1/3*2 vs. ADH3*2/3*2 had statistically equivalent demographic characteristics, drinking histories, and height-to-weight ratios, except that the ADH2*1/2*3 group reported more marijuana consumption than the ADH2*1/2*1 group (ADH2*1/2*1 mean = 0.98 ± 2.0, ADH2*1/2*3 mean = 13.2 ± 19.4, units are in times in the previous 6 months).
P3 amplitude was not found to vary as a function of NAH. In addition, no significant P3 amplitude differences were found as function of family history of alcohol dependence or ADH3 genotype. However, a significant relationship was found between ADH2 genotype and P3 amplitude. Higher amplitude P3a responses to the noise tone were observed in subjects with the ADH2*1/2*3 genotype in: FZ (F = 5.9; df = 1,45; p < .019) and CZ (F = 7.3; df = 1,45; p < .01), and trends were found for an increase in the P3b response to the noise tone in FZ (F = 3.29; df = 1,45; p < .07) and CZ (F = 3.05, df = 1,45; p < .09) and in P3a response to the rare tone in FZ (F = 3.7; df = 1,45; p < .06) and CZ (F = 3.07; df = 1,45; p < .09) as compared with subjects with the ADH2*1/2*1 genotype. Means for these variables are presented in Table 2.
ERP Response to Alcohol
The second major research question concerned whether significant effects of alcohol on P3 amplitude, as compared with placebo, could be found in these Native American men. Of the 47 men for whom placebo session ERP recordings were available, 42 completed both alcohol and placebo sessions. This group did not differ significantly on any of the demographic, or substance use variables from the group of 47 subjects presented in Table 1. Administration of 0.56 g/kg alcohol produced a mean ± SD (g/dL) BAC of 0.77 ± 0.02 at 60 min after beverage ingestion at the approximate time of ERP recordings. Table 3 presents BACs for each hypothesized risk group at 60 min; there were no significant differences in BACs between any of the risk groups (e.g., FHP vs. FHN).
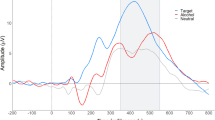
Alcohol produced a significant reduction in the P3a amplitude in response to the standard tone (drug: F = 5.33, df = 1,40; p < .028), rare (target) tone (drug: F = 13.9; df = 1,40; p < .001) and noise tone (drug: F = 13.5; df = 1,42; p < .001). An electrode effect was noted for the rare tone (electrode: F = 51.6; df = 2,82; p < .001) and noise tone (F = 38.4; df = 2,82; p < .001). However no drug × electrode interactions were found. Alcohol was not found to significantly alter the P3b amplitude. Grand averages of ERPs for the entire group of subjects (n = 42) for the alcohol and placebo condition are presented in Figure 1 .
Grand averages of ERPs elicited by an oddball plus noise paradigm in 42 Native American men at 60 minutes following placebo (left column) or alcohol (0.56 g/kg) (right column) ingestion. Averages are presented for frontal (FZ), central (CZ), and parietal (PZ) leads. N1 and P2, and P3 components are visible in response to standard tones (solid lines), rare, target tones (dotted lines), and noise bursts (dashed lines). Alcohol was found to significantly reduce the amplitude of the P3 component to all three tones.
Risk Factors and Response to Alcohol
The third major research question explored was whether hypothesized risk/protection factors (FH, NAH, ADH2 genotype, ADH3 genotype, low P3 amplitude at placebo) affected P3 amplitude in response to alcohol. Neither family history status, degree of Native American heritage, nor ADH3 genotype was associated with statistically significant difference in P3 response to alcohol. The ADH2 genotype × drug interaction, however, was found to be statistically significant as seen in Table 2. Men with ADH2*1/2*3 genotype exhibited greater alcohol-induced reductions in the P3a in CZ to the noise tone (F = 6.97; df = 1,40; p < .01) and the P3b in PZ to the rare tone (F = 3.93; df = 1,40; p < .05) than men with the ADH2*1/2*1 genotype. In addition, a trend toward greater P3a reductions following alcohol was found for the P3a component in lead PZ (F = 3.0; df = 1,40; p < .09).
Low (<10 microvolts) vs. normal (>10 microvolts) P3b amplitude at placebo in response to the noise tone was also found to be a predictor of response to alcohol. Differences in P3 amplitude response between the placebo and alcohol sessions were found to be significant between the low and average P3 groups for the P3a in FZ (F = 4.47; df = 1,40; p < .04), CZ (F = 4.35; df = 1,40; p < .04) and for the P3b in FZ (F = 8.62; df = 1,40; p < .005), CZ (F = 16.63; df = 1,40; p < .001), PZ (F = 10.4; df = 1,40; p < .003). A significant difference in response to the rare (target) tone was also found between groups for the P3b response in FZ (F = 5.6; df = 1,40; p < .02). As seen in Table 4, those subjects with average P3 amplitudes at placebo experienced reductions in P3 amplitude in the alcohol session, whereas those with low P3 amplitudes at placebo on average experienced either no response or an increase in P3 amplitude to alcohol challenge.
DISCUSSION
This study assessed the amplitude of the P3 component of the ERP under two conditions (60 min after placebo and 0.56 g/kg alcohol) using an auditory oddball plus noise paradigm in Native American Mission Indians of at least 25% NAH. The study was designed to explore determinants of P3 amplitude after placebo and alcohol in these Mission Indian men.
P3 amplitude is perhaps the most studied electrophysiological “marker” of potential vulnerability to alcohol dependence. Many studies, but not all, have supported the notion that the amplitude of the P3 is reduced in FHP subjects (see Elmasian et al. 1982; Begleiter et al. 1984; O'Connor et al. 1986, 1987; Whipple et al. 1988; Hill et al. 1988, 1990; Begleiter and Porjesz 1990; Porjesz and Begleiter 1990; Berman et al. 1993; Hill and Steinhauer 1993; Steinhauer and Hill 1993; Ramachandran et al. 1996). No previous studies have evaluated P3 amplitude in Native Americans, a group at particularly high risk for the development of alcoholism. No relationship was found between P3 amplitude and degree of NAH in this group of Mission Indian men. Additionally, Native American men with a family history of alcohol dependence did not significantly differ from those with no alcoholic relatives on P3 amplitude.
The lack of a clearly significant relationship between family history status and P3 amplitude in this group of Mission Indian men may be due to several factors. A meta-analysis in which P3 amplitude from 30 separate studies was analyzed, suggests that smaller P3 amplitudes were obtained from males with family histories of alcoholism compared with controls (Polich et al. 1994). However, a moderator analysis also indicated that ERP paradigms that used difficult visual tasks yielded the most reliable effects. Additionally, a higher mean effect size was found in studies that examined subjects who were younger than 18 years of age. In the present study, all subjects were 18 years or older and an easy auditory task was used. Thus, these two factors may have contributed to a lack of power to detect significant differences in the present study.
Another potential confounding factor in the present study concerns familial risk for alcoholism, which is clearly established in Euroamericans and some other ethnic groups, but has not yet been validated in Native American populations. Preliminary studies by Long et al. (1995) for instance, did not find familial risk for alcoholism in a Plains Indian tribe. They suggested that the lack of association of family history to risk for alcoholism in that population may be due to such factors as lack of power to detect familial association due to high prevalence of the disease, the possible presence of nonadditive genetic mechanisms, or equivalent high vulnerability to some environmental risk. The Native American men evaluated in the present study had a very high prevalence of family history of alcoholism (67.3%), consistent with research studies in other Native American tribes (Zeiner et al. 1985; Harford 1992; Long et al. 1995). Thus, the lack of a clear association between family history of alcoholism and P3 amplitude, in the present study, remains difficult to interpret if familial risk itself has not been substantiated among Native American populations.
Another approach to understanding potential factors associated with risk for the development of alcohol dependence has been to focus on response to alcohol. The results of this study replicate previous findings that P3 amplitude of the ERP is sensitive to the effects of alcohol (see Roth et al. 1977; Porjesz and Begleiter 1985; Campbell and Lowick 1987; Porjesz et al. 1987). A reduction in the P3a component across the scalp was the most prominent effect of alcohol in these Native American men. Studies evaluating the response of P3 to differences in stimuli have suggested that at least two components of the P3 can be recorded from the cortical surface (Courchesne et al. 1975; Squires et al. 1975; Beck et al. 1980; Knight 1984; Yamaguchi and Knight 1991). P3s that are generated by stimuli that are task relevant and correctly detected by the subject, appear to be of maximal voltage over parietal cortex, and have been designated the “target P3” or P3b. Whereas, nontarget stimuli that are “unexpected” or “novel” but require no behavioral response, appear to generate an earlier latency potential that may be more frontocentral in origin, designated the “novelty P3” or P3a (Roth et al. 1984; Halgren et al. 1986; Polich and Bloom 1987; Polich 1988). In addition, P3a components have also been specifically noted to occur in paradigms where the detection of an infrequent target stimulus was required when nontarget, infrequent novel stimuli, and relatively frequent standard stimuli are both presented (Courchesne et al. 1975, 1977) as was the case in the present study. In several studies, alcohol has been shown to reduce the amplitude of the P3 preferentially to “novel” or distracting stimuli as opposed to target stimuli (Campbell and Lowick 1987; Grillon et al. 1995). Thus, the finding of reduced P3a amplitude following alcohol challenge in the present study is consistent with the literature and suggests that aspects of stimulus evaluation that involve the novelty or orienting may be more affected by alcohol in this population. The fact that no effects of alcohol were found on the P3b component of the ERP further suggests that these Mission Indian men may be less sensitive, as a whole, to the effects of alcohol on task-relevant aspects of stimuli. These data are consistent with previous studies in this population demonstrating that these Mission Indian men may have a less intense response to alcohol as quantified by subjective and other objective measures of intoxication (Ehlers et al. 1997; Garcia-Andrade et al. 1997).
The establishment of a particular measure as a risk factor for the development of alcohol dependence requires not only that it exists in persons at increased risk for developing the disorder, but that those persons identified by the measure actually go on to develop alcohol problems. In the case of lowered P3 amplitude and alcohol dependence, longitudinal studies have yet to establish this relationship. However, one variable that has not been established, not only as being present in high-risk individuals, but is also predictive of alcohol problems on 8-year follow-up, is low level of response to alcohol (see Schuckit and Smith 1996). In that study, low level of response to alcohol in men 18–25 years of age was predictive of later life alcohol problems in and of itself, independent of family history status and drinking practices at the time of the original study.
The relationship between P3 amplitude at baseline and response to alcohol challenge has not been previously discussed. In the present study, lower P3 amplitude in the placebo session, independent of family history or NAH, was found to be predictive of less reduction or an actual increase in P3a and P3b amplitudes following alcohol challenge. Thus alcohol may actually be acting to “normalize” P3 amplitude in some individuals with low P3 amplitudes at baseline. These findings suggest that P3 amplitude may be a risk “marker” for a less intense response to alcohol, a variable that has been clearly established to be predictive of the development of problem drinking (Schuckit and Smith 1996). This finding of less alcohol-induced impairment in stimulus evaluation in Mission Indian men with lowered P3 amplitudes, although interesting, and consistent with recent hypotheses in alcohol research, needs to be replicated in larger and more ethnically diverse populations before its relationship to risk for alcohol dependence can be clearly established.
Other variables that have also been established as influencing the development of alcohol dependence are genetic polymorphisms in alcohol metabolizing enzymes. For example, among individuals of Asian heritage (i.e., Chinese and Japanese) distinct differences in rates of alcoholism are associated with polymorphisms in the aldehyde dehydrogenase (ALDH2) gene (Thomasson et al. 1991; Higuchi et al. 1995). Individuals who are homozygous for the ALDH2*2 allele represent approximately 10% of Chinese and Japanese populations, but no alcoholics have been found with this genotype. Individuals who are heterozygous for the ALDH2*2 allele account for approximately 35% of Chinese and Japanese populations, but only 12% of Japanese and Chinese alcoholics have the heterozygous ALDH2*2 genotype. Additionally, genetic polymorphisms in ADH appear to interact with the ALDH2*2 gene to influence alcohol dependence. Lower frequencies of both ADH2*2 and ADH3*1 alleles (in addition to the lower incidence of ALDH2*2 alleles) have also been found among Japanese and Chinese alcoholics compared with nonalcoholic controls (Thomasson et al. 1991; Chao et al. 1994; Higuchi et al. 1995).
Given that a low response to alcohol has been shown to be a risk factor for developing alcohol-related problems in the future (Schuckit and Smith 1996), it is also of interest to understand how these gene polymorphisms affect intensity of response to alcohol. For example, Asian subjects who are heterozygous for the ALDH2*2 allele demonstrate more intense objective and subjective responses to alcohol (Wall et al. 1992, 1993, 1994) including greater alcohol-induced reductions in P3 amplitude (Wall and Ehlers 1995). Thus, Asians with ALDH2*2 alleles may be at lower risk for developing alcoholism inasmuch as they experience a more intense reaction to alcohol, and thus are less likely to drink heavily. The population of Mission Indian men in the current study do not have ALDH2*2 alleles (Wall et al. 1997), but results indicate that the ADH2*3 allele is associated with higher amplitude P3 components following placebo and is also associated with greater alcohol-induced reductions in P3 amplitude in this population. Thus, these data suggest this allele may be associated with a less intense response to alcohol and as such may be a protective factor against the development of alcoholism in this population. One confounding variable, however, was that the men with ADH2*3 alleles used more marijuana in the previous 6 months than the men with ADH2*1 alleles. Although this could theoretically influence P3 amplitude or response to alcohol, Patrick et al. (1995) have found that chronic marijuana usage had no effect on the amplitude or latency of the P3 in subjects without medical and psychiatric diagnoses. Additionally, more recent studies by Patrick et al. (1997) have demonstrated that early and middle latency evoked potentials are also unaltered in medically and psychiatrically normal daily marijuana users.
In summary, these results suggest the presence of certain biological variables within this population of Mission Indian men that may be associated with the future development of alcohol-related problems. Longitudinal studies are needed, however, to establish whether P3 amplitude or ADH2 genotype is associated with risk or protection from the development of alcoholism or other alcohol-related disease later in life.
References
American Psychiatric Association. (1980): Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Washington, DC, APA
Andre JM . (1979): The Epidemiology of Alcoholism among American Indians and Alaska Natives. US Indian Health Services, Albuquerque, NM, U.S. Government Printing Office
Beck EC, Swanson C, Dustman RE . (1980): Long latency components of the visual evoked potentials in man: Effects of aging. Exp Aging Res 6: 523–545
Begleiter H, Porjesz B . (1990): Event-related potentials in populations at risk for alcoholism. EEG Clin Neurophysiol Suppl 41: 177–182
Begleiter H, Porjesz B, Bihari B, Kissin B . (1984): Event-related brain potentials in boys at risk for alcoholism. Science 225: 1493–1496
Berman SM, Martinez RA, Noble EP . (1993): Familial alcoholism and ERPs: Differences in probability sensitivity? Alcohol Alcohol 28: 695–707
Bosron WF, Ehrig T, Li T-K . (1993): Genetic factors in alcohol metabolism and alcoholism. Semin Liver Dis 13: 126–135
Brod TM . (1975): Alcoholism as a mental health problem of Native Americans. Arch Gen Psychiatry 32: 1385–1391
Cahalan D . (1970): Problem Drinkers. San Francisco, CA, Jossey-Bass Inc.
Campbell KB, Lowick BM . (1987): Ethanol and event-related potentials: The influence of distracter stimuli. Alcohol 4: 257–263
Chao Y-C, Kiou S-R, Chung Y-Y, Tang H-S, Hsu C-T, Li T-K, Yin S-J . (1994): Polymorphism of alcohol and aldehyde dehydrogenase genes and alcoholic cirrhosis in Chinese patients. Hepatology 19: 360–366
Chavetz ME . (1964): Consumption of alcohol in the far and middle East. N Engl J Med 217: 297–301
Courchesne E, Hillyard S, Courchesne R . (1977): P3 waves to the discrimination of targets in homogeneous and heterogeneous stimulus sequences. Psychophysiology 14: 590–597
Courchesne E, Hillyard SA, Galambos R . (1975): Stimulus novelty, task relevance and the visual evoked potential in man. EEG Clin Neurophysiol 39: 131–143
Couzigou P, Fleury B, Groppi A . (1990): The French Group for Research on Alcohol and Liver Genotyping study of alcohol dehydrogenase class I polymorphism in French patients with alcoholic cirrhosis. Alcohol Alcohol 25: 623–626
Day CP, Bashir R, James OFW, Bassendine MF, Crabb DW, Thomasson HR, Li T-K, Edenberg HJ . (1991): Investigation of the role of polymorphisms at the alcohol and aldehyde dehydrogenase loci in genetic predisposition to alcohol-related end-organ damage. Hepatology 14: 798–801
Ehlers CL, Garcia-Andrade C, Wall T, Cloutier D, Phillips E . (1997): EEG response to ethanol in Native American Mission Indians. Biological Psychiatry, in press.
Ehlers CL, Phillips E, Parry BS . (1996): Electrophysiological findings during the menstrual cycle in women with and without late luteal phase dysphoric disorder: Relationship to risk for alcoholism. Biol Psychiatry 39: 720–732
Elmasian R, Neville H, Woods D, Schuckit M, Bloom FE . (1982): Event-related brain potentials are different in individuals at high and low risk for developing alcoholism. Proc Natl Acad Sci USA 79: 7900–7903
Garcia-Andrade C, Wall TL, Ehlers CL . (1996): Alcohol expectancies in a Native American population. Alcohol Clin Exp Res 20 (8): 1438–1442
Garcia-Andrade C, Wall TL, Ehlers CL . (1997): The firewater myth and response to alcohol in Mission Indians. Am J Psychiatry 154: 983–988
Gilder JF, Hodgkinson S, Murray RM . (1993): ADH and ALDH genotype profiles in Caucasians with alcohol-related problems and controls. Addiction 88: 383–388
Grillon C, Sinha R, O'Malley SS . (1995): Effects of ethanol of the processing of low probability stimuli: An ERP study. Psychopharmacology 119: 455–465
Halgren E, Stapleton JM, Smith M, Altafullah I . (1986): Generators of the human scalp P3 (s). In Cracco RQ, Bodis-Wollner (eds), Evoked Potentials, New York, Liss, pp 269–284
Harford TC . (1992): Family history of alcoholism in the United States: Prevalence and demographic characteristics. Br J Addict 87: 931–935
Helzer JE, Canino GJ, Yeh E-K, Bland RC, Lee CK, Hwu H-G, Newman S . (1990): Alcoholism—North America and Asia. Arch Gen Psychiatry 47: 313–319
Higuchi S, Matsushita S, Murayama M, Takagi T, Hayashida M . (1995): Alcohol and aldehyde dehydrogenase polymorphisms and the risk for alcoholism. Am J Psychiatry 152: 1219–1221
Hill SY, Steinhauer S . (1993): Assessment of prepubertal and post-pubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. J Stud Alcohol 54: 350–358
Hill SY, Steinhauer S, Park J, Zubin J . (1990): Event-related potentials characteristics in children of alcoholics from high density families. Alcohol Clin Exp Res 14: 6–17
Hill SY, Steinhauer S, Zubin J, Baughman T . (1988): Event-related potentials as markers for alcoholism risk in high density families. Alcohol Clin Exp Res 12: 545–554
Klatsky AL, Sieglaub AB, Lanay C, Friedman GD . (1983): Racial patterns of alcoholic beverage use. Alcohol Clin Exp Res 7: 372–377
Knight RT . (1984): Decreased response to novel stimuli after prefrontal lesions in man. EEG Clin Neurophysiol 54: 9–20
Knupfler G, Room R . (1967): Drinking patterns and attitudes of Irish, Jewish, and white Protestant American men. Q J Stud Alcohol 28: 676–699
Kunitz SJ, Levy J, Odoroff C, Bollinger J . (1971): The epidemiology of alcoholic cirrhosis in two Southwestern Indian tribes. Q J Stud Alcohol 1971: 706–720
Lemert EW . (1964): Forms and pathology of drinking in three Polynesian societies. Am Anthropol 66: 361–374
Lin TV . (1953): A study of the incidence of mental disorder in the Chinese and other cultures. Psychiatry 16: 313–336
Long IC, Robin R, Washa J, Brown G, Goldman D . (1995): Lack of familial alcoholism in an American Indian sample. Alcohol Clin Exp Res 19: 71A
May PA . (1982): Substance abuse and American Indians: Prevalence and susceptibility. Int J Addict 17: 1185–1209
McGarver-May DG, Thomasson H, Martier SS, Sokol R, Li T-K . (1996): Alcohol dehydrogenase 2*3 allele protects against adverse developmental offspring outcome after intrauterine alcohol exposure. Alcohol Clin Exp Res 20: 122A
Mendelson JH, McGuire M, Mello NK . (1984): A new device for administering placebo alcohol. Alcohol 1: 417–419
Monteiro MG, Schuckit MA . (1989): Alcohol, drug, and mental health problems among Jewish and Christian men at a University. Am J Drug Alcohol Abuse 15: 403–412
Newlin DB, Thomson JB . (1990): Alcohol challenge with sons of alcoholics: A critical review. Psychol Bull 108: 383–402
O'Connor S, Hesselbrock V, Tasman T . (1986): Correlates of increased risk for alcoholism in young men. Prog Neuropsychopharmacol Biol Psychiatry 10: 211–218
O'Connor S, Hesselbrock V, Tasman T, DePalma N . (1987): P3 amplitude in two distinct tasks are decreased in young men with a history of paternal alcoholism. Alcohol 4: 323–330
Patrick G, Straumanis JJ, Struve FA, Nixon F, Fitz-Gerald MJ, Manno JE, Soucair M . (1995): Auditory and visual P300 event related potentials are not altered in medically and psychiatrically normal chronic marijuana users. Life Sci 56: 2135–2140
Patrick G, Straumanis JJ, Struve FA, Fitz-Gerald MJ, Manno JE . (1997): Early and middle latency evoked potentials in medically and psychiatrically normal daily marihuana users: a paucity of significant findings. Clin Electroencephalogr 28: 26–31
Polich J . (1988): Bifurcated P300 peaks: P3a and P3b revisited. J Clin Neurophys 5: 287–294
Polich J, Bloom FE . (1987): P300 from normals and adult children of alcoholics. Alcohol 4: 301–305
Polich J, Fischer A, Starr A . (1983): A programmable multi-tone generator. Behav Res Methods Instrum 15: 39–41
Polich J, Pollock VE, Bloom FE . (1994): Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull 115: 55–73
Pollock VE . (1992): Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Am J Psychiatry 149: 1534–1536
Porjesz B, Begleiter H . (1985): Human brain electrophysiology and alcoholism. In Tarter RE, Van Thiel D (eds), Alcohol and the Brain. New York. Plenum Press, pp 139–182
Porjesz B, Begleiter H . (1990): Event-related potentials in individuals at risk for alcoholism. Alcohol 7: 465–469
Porjesz B, Begleiter H, Bihari B, Kissin B . (1987): Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol 4: 283–287
Ramachandran G, Porjesz B, Begleiter H, Litke A . (1996): A simple auditory oddball task in young adult males at high risk for alcoholism. Alcohol Clin Exp Res 20: 9–15
Roth WT, Dorato KH, Kopell BS . (1984): Intensity and task effects on evoked physiological responses to noise bursts. Psychophysiology 21: 466–481
Roth WT, Tinkleberg JR, Kopell BS . (1977): Ethanol and marijuana effects on event-related potentials in a memory retrieval paradigm. EEG Clin Neurophysiol 42: 381–388
Schuckit MA . (1984): Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry 41: 879–884
Schuckit MA . (1985): The clinical implications of primary diagnostic groups among alcoholics. Arch Gen Psychiatry 42: 1043–1049
Schuckit MA, Gold EO . (1988): A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch Gen Psychiatry 45: 211–216
Schuckit MA . (1994): A clinical model of genetic influence in alcohol dependence. J Stud Alcohol 55: 5–17
Schuckit MA, Smith TL . (1996): An 8-year follow-up of 450 sons of alcoholics and controls. Arch Gen Psychiatry 53: 202–210
Singer K . (1972): Drinking patterns and alcoholism in the Chinese. Br J Addict 67: 3–14
Squires NK, Squires KC, Hillyard SA . (1975): Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. EEG Clin Neurophysiol 38: 387–401
Steinhauer SR, Hill SY . (1993): Auditory event-related potentials in alcoholics and their first-degree relatives. J Stud Alcohol 54: 408–421
Thomasson HR, Edenberg HJ, Crabb DW, Mai X-L, Jerome RE, Li T-K, Wang S-P, Lin Y-T, Lu R-B, Yin S-J . (1991): Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. Am J Hum Genet 48: 667–681
U.S. Indian Health Service (1977): Alcoholism: A High Priority Health Problem. Washington, DC, U.S. Government Printing Office
U.S. Indian Health Service (1982): Analysis of Fiscal Year 1981 Indian Health Service and U.S. Hospital Discharge Rates by Age and Primary Diagnosis. Washington, DC, U.S. Government Printing Office
Wall TL, Ehlers CL . (1995): Acute effects of alcohol on P300 in Asians with different ALDH2 genotypes. Alcohol Clin Exp Res 19: 617–622
Wall TL, Gallen CC, Ehlers CL . (1993): Effects of alcohol on the EEG in Asian men with genetic variations of ALDH2. Biol Psychiatry 34: 91–99
Wall TL, Garcia-Andrade C, Thomasson HR, Cole M, Ehlers CL . (1996): Alcohol elimination in Native American Mission Indians: An investigation of interindividual variation. Alcohol Clin Exp Res 20: 1159–1164
Wall TL, Garcia-Andrade C, Thomasson HR, Ehlers CL . (1997): Alcohol dehydrogenase polymorphisms in Native Americans: Identification of the ADH2*3 allele. Alcohol Alcohol 32: 129–131
Wall TL, Nemeroff CB, Ritchie J, Ehlers CL . (1994): Cortisol responses following placebo and alcohol in Asians with different ALDH2 genotypes. Alcohol 55: 207–213
Wall TL, Thomasson HR, Schuckit MA, Ehlers CL . (1992): Subjective feelings of alcohol intoxication in Asians with genetic variations of ALDH2 alleles. Alcohol Clin Exp Res 16: 991–995
Whipple S, Parker E, Nobel E . (1988): An atypical neurocognitive profile in alcoholic fathers and their sons. J Stud Alcohol 49: 240–244
Xu Y, Carr LG, Bosron WF, Li T-K, Edenberg HJ . (1988): Genotyping of human alcohol dehydrogenase at the ADH2 and ADH3 loci following DNA sequence amplification. Genomics 2: 209–214
Yamaguchi S, Knight RT . (1991): Anterior and posterior association cortex contributions to the somatosensory P300. J Neurosci 11: 2039–2054
Zeiner AR, Girardot JM, Jones-Saumty D, Nichols N . (1985): Prevalence of ALDH1 isoenzyme among American Indians in Oklahoma. Jpn J Alcohol Stud Drug Depend 20: 359–366
Acknowledgements
This work was presented in part at the 1997 Research Society for Alcoholism meeting and was supported in part by the National Institute of Alcoholism and Alcohol Abuse Grants: 00155, 00223, 04620, 10201, General Clinical Research Center Grant 00833, and supported by the Office of Minority Health.
The authors would like to thank Susan Lopez and David Cloutier for assistance in data analysis and Diane Braca for editorial assistance. The computer programs for ERP analysis were written by Dr. James Havstad. The ADH genotyping was performed in the laboratories of Dr. Holly Thomasson and Dr. Lucinda Carr, Indiana University School of Medicine, Indianapolis, IN.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ehlers, C., Garcia-Andrade, C., Wall, T. et al. Determinants of P3 Amplitude and Response to Alcohol in Native American Mission Indians. Neuropsychopharmacol 18, 282–292 (1998). https://doi.org/10.1016/S0893-133X(97)00160-7
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(97)00160-7
Keywords
This article is cited by
-
PSPH-D-18-00526: Effect of a dual orexin receptor antagonist (DORA-12) on sleep and event-related oscillations in rats exposed to ethanol vapor during adolescence
Psychopharmacology (2020)
-
Association between single nucleotide polymorphisms in the mu opioid receptor gene (OPRM1) and self-reported responses to alcohol in American Indians
BMC Medical Genetics (2008)
-
Genomic screen for loci associated with tobacco usage in Mission Indians
BMC Medical Genetics (2006)