Abstract
The therapeutic effects of chronic selective serotonin reuptake inhibitors (SSRIs) are well documented, yet the elementary behavioral processes that are affected by such treatment have not been fully investigated. We report here the effects of chronic fluoxetine treatment and genetic deletion of the serotonin transporter (SERT) on food reinforced behavior in three paradigms: the progressive ratio operant task, the concurrent choice operant task, and the Pavlovian-to-Instrumental transfer task. We consistently find that chronic pharmacological blockade or genetic deletion of SERT result in similar behavioral consequences: reduced operant responding for natural reward. This is in line with previous studies reporting declines in operant responding for drugs and intracranial self-stimulation with fluoxetine treatment, suggesting that the effect of SERT blockade can be generalized to different reward types. Detailed analyses of behavioral parameters indicate that this reduction in operant responding affect both goal-directed and non-goal-directed behaviors without affecting the Pavlovian cue-triggered excessive operant responding. In addition, both pharmacological and genetic manipulations reduce locomotor activity in the open field novel environment. Our data contrast with the effect of dopamine in increasing operant responding for natural reward specifically in goal-directed behaviors and in increasing Pavlovian cue-triggered excessive operant responding. Serotonin and dopamine have been proposed to serve opposing functions in motivational processes. Our data suggest that their interactions do not result in simple opponency. The fact that pharmacological blockade and genetic deletion of SERT have similar behavioral consequences reinforces the utility of the SERT null mice for investigation of the mechanisms underlying chronic SSRIs treatment.
Similar content being viewed by others
INTRODUCTION
Selective serotonin reuptake inhibitors (SSRIs) are the most frequently prescribed antidepressants; they display therapeutic effects when used chronically, but not acutely. The main site of action of SSRIs is thought to be the serotonin transporter (SERT), which is responsible for reuptake and recycling of released serotonin (Hirschfeld, 2000; Owens, 2004). Blockade of SERT by SSRIs and the resultant elevation in extracellular serotonin levels are thought to mediate the therapeutic effects of SSRIs (Bergqvist et al, 1999; Cryan et al, 2005). Yet the underlying neurobiological mechanisms and elementary behavioral processes associated with SSRIs' apparent therapeutic or side effects have not been fully investigated.
One of the elementary behavioral processes that might be affected by SSRIs is motivation, which can be operationally defined as an animal's willingness to expend energy to obtain a reward or avoid punishment. Many studies have investigated the effects of SSRIs on operant responses in intracranial self-stimulation (ICSS) or drug self-administration paradigms. The most consistent finding was that SSRIs reduced responding for these reinforcers, or shifted it in ways that indicated reductions in motivation (Carroll et al, 1990; Richardson and Roberts, 1991; Tella, 1995; Lee and Kornetsky, 1998; Wilson et al, 2000; Harrison et al, 2001; Harrison and Markou, 2001). However, there are limited studies that examine the influence of SSRIs on responding for natural rewards such as food. Studies that use natural rewards have mostly focused on the role of serotonin in impulsivity (Bizot et al, 1999; Joel et al, 2004; Winstanley et al, 2004) rather than on motivation or reinforcement learning processes.
In addition to SERT pharmacologic blockade, mice with a genetic deletion of the SERT have been generated. They display molecular changes similar to those observed with chronic SSRI treatment, although to a significantly greater magnitude (Bengel et al, 1998; Sora et al, 1998; Lira et al, 2003). Studies on the involvement of serotonin in reinforced behavior in SERT-deficient mice are also mainly limited to the context of drug use. Cocaine-induced place preference is maintained in SERT-deficient mice (Sora et al, 1998). These mice are also anxious, hypoactive and less aggressive (Holmes et al, 2002a, 2002b, 2003a, 2003b, 2003c; Ansorge et al, 2004). However, little is known about the effects of SERT deletion on responses for natural reward.
We report here the effects of chronic fluoxetine treatment and genetic deletion of the SERT on food-reinforced behavior in three paradigms: first, the progressive ratio operant task (PR), which tests the amount of work an animal is willing to perform for food reward (Hodos, 1961); second, the concurrent food choice operant task, which allows a more careful analysis of effort expended to obtain a preferred reward (Cousins and Salamone, 1994); and third, the Pavlovian-to-Instrumental transfer task (PIT), which examines the ability of Pavlovian conditioned cues that predict reward to enhance operant responding (Wyvell and Berridge, 2000). Our results indicate that both fluoxetine treatment and SERT gene deletion reduce operant responding for natural reward as well as a generalized reduction in motor output.
MATERIALS AND METHODS
Animals
All animals were group-housed (4–5 /cage) in a temperature- and humidity-controlled (25°C) barrier facility, with lights on/off at 0600/1800 h. All testing was conducted during the light phase with the exception of wheel running activity which is tested continuously for 2 weeks. All animal procedures were approved by the Institutional Animal Care and Use Committee at The University of Chicago.
SERT null mice were generated by insertion of the Cre recombinase cDNA and polyA into the 5′-UTR of SERT genomic DNA. The generation and use of this line as a tissue-specific Cre recombinase expression line was described elsewhere (Zhuang et al, 2005). Insertion of Cre resulted in the complete absence of SERT mRNA and protein, which was confirmed by in situ hybridization and ligand binding autoradiography respectively (not shown). These mice were originally generated on a 129SvJ background, and were subsequently crossed to C57BL6/J for four generations. All SERT null and wild-type (WT) mice were generated by breeding heterozygotes, thus permitting the use of littermate controls. The same group of SERT null and WT (N=12 per genotype, equal number of males and females) mice were used for progressive ratio and for the concurrent choice task. A different group (N=12 per genotype, equal number of males and females) was used for the Pavlovian to Instrumental Transfer task (PIT).
The fluoxetine experiments utilized all male C57BL/6J mice (ordered from the Jackson Laboratory). A first group of mice (N=12 per treatment) was used for the progressive ratio sated condition. A second independent group (N=12 per treatment) was used for the progressive ratio food-deprived condition. A third independent group (N=12 per treatment) was used for PIT and subsequently for the concurrent choice task.
The above animal numbers reflect the total number of animals with which we began the experiment; due to deaths during the lengthy experiment or failure to learn operant tasks, the actual numbers as reflected in figure legends may be slightly different.
Drugs
Fluoxetine hydrochloride (generously provided by NIMH) was dissolved in dH2O and administered at a dose of 10 mg/kg, in a volume of 0.1 ml/10 g body weight. IP injections of either fluoxetine or 0.9% saline were given after the daily behavioral sessions, to ensure that chronic rather than acute drug effects were examined, with the exception of the first dose, which was administered before the daily behavioral session.
Progressive ratio data collection started at the beginning of fluoxetine treatment and lasted for 3 weeks. As the same treatment effect was seen throughout the 3 weeks, all 3 weeks were used in data analysis. For PIT, mice had been treated with daily fluoxetine for 2 weeks before Pavlovian training, which continued throughout the experiment. For the concurrent choice task, which was conducted after PIT in the same group of animals, mice had been treated with fluoxetine for 8 weeks before the experiment, which continued throughout the experiment. Body weights were taken during the progressive ratio sated condition after 3 weeks of fluoxetine treatment. Open-field locomotor activity was measured after completion of progressive ratio experiments (after 3–4 weeks of fluoxetine treatment, as indicated below).
Progressive Ratio Operant Task
The progressive ratio (PR) operant task was conducted in six mouse operant conditioning chambers with two retractable levers on either side of the feeder, a house light, two signal lights above the levers, and a feeder with photobeam (Med Associates, St Albans, VT). All mice were maintained at 85–90% of their free-feeding body weight except during the food-sated testing condition. Mice were given five 45-min sessions per week, and were trained as follows: 2 days of magazine training in which one pellet was delivered with a variable interval of 30 s that was independent of the animal's responses, followed by fixed-ratio 1 (FR1) sessions until animals reached criteria of 30 or more lever presses over 2 consecutive days. When all animals acquired lever pressing on an FR1 schedule, which took varying lengths of time, a final FR1 session was given to all animals, and progressive ratio testing commenced. An inactive lever was used to control for non-specific activity. Food-sated animals were run on a PR3 schedule (increment by three lever presses after each reward); food-deprived, a PR5 schedule (increment by five lever presses after each reward). Breakpoint was defined either as the last ratio completed in the 45-min session, or the last ratio completed before the animal stopped lever pressing for 5 min (which terminated the session). The two fluoxetine progressive ratio experiments (food-sated and food-deprived) therefore each involved two phases: 3 weeks of baseline progressive ratio sessions, followed by 3 weeks of treated progressive ratio sessions in either food-deprived or food-sated conditions.
Pavlovian to Instrumental Transfer
These sessions were adapted from the protocol published by Wyvell and Berridge (2000). Animals were maintained at 85–90% of their free-feeding body weight. Animals were trained with 30-min sessions of variable interval (VI) schedules, which were increased from VI 10 s (VI10) to VI30. Once the VI30 schedule was reached, baseline measurements were taken for 2 weeks. Two control sessions were then given before commencement of Pavlovian training sessions. The control sessions consisted of the normal VI30 session run in the dark, with a light randomly activated for 30 s, five times during the session. Control sessions were to examine the possibility that any observed response rate changes during light presentation was to a non-specific effect of light. Mice were then given 10 Pavlovian conditioning sessions, with no levers present. The Pavlovian cue was identical to the cue used during the control sessions and was presented 10 times randomly distributed in the 30-min session. The cue concluded with delivery of a food pellet. Following the 10 Pavlovian sessions (a total of 100 light-food parings), animals were given two normal VI30 sessions to restore lever-pressing behavior. Four test sessions were then conducted under extinction conditions. Test sessions contained five 30 s periods of illumination of the Pavlovian light cue which were randomly distributed in the 30 min session. Analysis consisted of comparison of the press rate during the 2.5 min before the light was illuminated (baseline) relative to the press rate during the 2.5 min of illumination. Data are presented as enhancement ratio: ‘presses during light/(presses during baseline+presses during light).’ An enhancement ratio greater than 0.5 indicates lever pressing is enhanced by the cue.
The Concurrent Food Choice Operant Task
This behavioral paradigm was adapted from Cousins and Salamone, (1994). The concurrent choice task was carried out following the PIT sessions (see above). Animals were trained to lever press as discussed above under PIT, and maintained at 85–90% of their free-feeding body weight. The task consisted of five 30-min FR 20 (for the fluoxetine vs saline experiment) or FR 30 (for the SERT null vs WT mice experiment) sessions. During 3 of these sessions (on alternate days), regular rodent chow was available in the operant chambers along with the operant levers (‘choice’ condition). The other 2 days were the ‘no choice’ condition, in which rodent chow was not available, and food could be obtained only by lever pressing.
Open Field Locomotor Activity
Each mouse was placed in an acrylic open field chamber 40 cm long × 40 cm wide × 37 cm high (Med Associates, St Albans, VT). No background noise was provided and illumination was set to 20–25 lux. Infrared beams recorded the animal's location and path (locomotor activity). Animals were placed in the lower right-hand corner of the open field and their activity was measured for 30 min. Data are presented as total distance traveled during the entire session. The chambers were cleaned with 90% EtOH between all sessions.
Home Cage Wheel Running Activity
Mice were singly housed each with a 4.5-inch wire mesh wheel (Pets International, Ltd). Two counterbalanced magnets (Digi-key) were attached to the wheel. The wheel was situated in the cage such that a magnetic switch closes (Digi-key) at every pass of a magnet. Data were collected using Vitalview acquisition software, QA-4 activity input modules and DP-24 data ports (Mini-mitter). Data were collected every 5 min for 2 weeks. The total number of bouts in 2 weeks, the average revolutions per bout, and the average wheel running speed were recorded. A bout was defined as consecutive minutes where the mouse turned the wheel minimally 2.5 complete revolutions.
Data Analysis
Data were analyzed using StatView 5.0.1. Independent two-tailed Student t-test was used when genotype or treatment was the only grouping variable. ANOVA was used when additional factors were considered. Repeated measures ANOVA was used when data were collected in multiple trials.
RESULTS
Blockade or Deletion of SERT Reduced Operant Responding for Food Reward
We measured the break point in the progressive ratio operant task, which is considered to be an index of how hard the animal is willing to work for rewards (Hodos, 1961). Overall, SERT null mice had lower break points, indicating a reduced motivation to work for reward. This was true for both food-deprived (genotype effect in repeated measures ANOVA, Figure 1a, F(1,21)=4.5, p<0.05) and food-sated conditions (Figure 1b, F(1,20)=5.8, p<0.05).
Both genetic deletion and pharmacological blockade of the SERT reduced operant responding in progressive ratio (PR) operant tasks. (a) Food-deprived SERT null mice displayed lower break point in PR5 than WT controls (N=11 for SERT null group, N=12 for WT group, F(1,21)=4.5, p<0.05). (b) Food-sated SERT null mice displayed significantly lower break point in PR3 than WT controls (N=10 for SERT null, N=12 for WT, F(1,20)=5.8, p<0.05). (c) Food-deprived fluoxetine-treated animals displayed significantly lower break point in PR5 than saline controls (N=11 for fluoxetine group, N=12 for saline group, F(1,21)=4.9, p<0.05). (d) Food-sated fluoxetine-treated animals displayed significantly lower break point in PR3 than saline controls (N=12 per group, F(1,22)=6.4, p<0.05).
Similarly, fluoxetine treatment caused a significant decrease in break point in the progressive ratio operant task, which was present regardless of feeding state (treatment effect in repeated measures ANOVA, Figure 1c, F(1,21)=4.9, p<0.05 for food-deprived and Figure 1d, F(1,22)=6.4, p<0.05 for food-sated conditions).
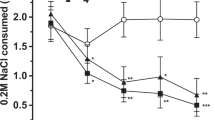
To examine food-motivated behavior in more detail, we subjected food-deprived animals to the concurrent food choice operant task, in which animals could lever-press for a preferred reward, or eat standard rodent chow that was freely available in the operant chamber. SERT null mice displayed significantly lower operant responding during ‘no choice’ days (genotype effect in repeated measures ANOVA, Figure 2b, F(1,20)=4.4, p<0.05) as well as a trend toward lower responding on ‘choice’ days (Figure 2a, F(1,20)=4.0, p=0.059). There was a trend toward a reduction in the percentage of food obtained from lever pressing in SERT null mice (genotype effect in repeated measures ANOVA, Figure 2c, F(1,20)=4.1, p=0.056). However, total food consumption was not significantly different between the two genotypes (genotype effect in repeated measures ANOVA, Figure 2d, F(1,20)=2.2, p>0.1), neither was chow consumption during the choice condition (genotype effect in repeated measures ANOVA, Figure 2e, F(1,20)=1.8, p>0.1).
The effects of genetic deletion and pharmacological blockade of SERT in the concurrent choice operant task. (a) There was a tendency for SERT deletion to reduce lever presses in the ‘choice’ condition (N=10 for SERT null, N=12 for WT, F(1,20)=4.0, p=0.059). (b) SERT deletion significantly reduced lever presses in the ‘no choice’ condition (F(1,20)=4.4, p<0.05). (c) There was a tendency for SERT deletion to reduce the percentage of food obtained from lever pressing (F(1,20)=4.1, p=0.056). (d) There was no difference in total food consumed between SERT null and WT mice (F(1,20)=2.2, p>0.1). (e) There was no genotype effect on chow consumption during the choice condition (F(1,20)=1.8, p>0.1). (f) Fluoxetine treatment did not affect lever presses in the ‘choice’ condition (N=12 for fluoxetine and N=11 for saline, F(1,21)=1.2, p>0.2). (g) Fluoxetine treatment did not affect lever presses in the ‘no choice’ condition (F(1,21)=1.5, p>0.2). (h) Fluoxetine treatment did not affect the percentage of food obtained from lever pressing (F(1,21)=0.03, p>0.5). (i) There was a tendency toward reduction of total food consumption the fluoxetine-treated group (F(1,21)=4.0, p=0.06). (j) There was no treatment effect on chow consumption during choice condition (F(1,21)=2.8, p>0.1).
Fluoxetine treatment did not significantly alter any of the parameters measured in the concurrent choice task (treatment effect in repeated measures ANOVA, Figure 2e, f, g and h, p>0.1 for all). Although there was a trend toward a reduction in total food consumed (treatment effect in repeated measures ANOVA, Figure 2h, F(1,21)=4.0, p=0.06), there was no difference in chow consumption during choice condition (treatment effect in repeated measures ANOVA, Figure 2j, F(1,21)=2.8, p>0.1).
In addition to unaffected food consumption in the above experiments, we did not observe changes in body weight in SERT null mice (genotype effect in two-way ANOVA, Figure 3a, F(1,18)=0.74, p>0.3) or after chronic fluoxetine treatment (treatment effect in unpaired t-test, Figure 3b, t(22)=1.3, p>0.2), further reinforcing the conclusion that reduced operant responding for food reward was not due to appetite suppression in the SERT null or fluoxetine-treated mice.
Neither genetic deletion nor pharmacological blockade of SERT affected body weight. (a) SERT deletion did not affect body weight (five SERT null female, five SERT null male, six WT female, six WT male, F(1,18)=0.74, p>0.3). (b) Chronic SERT blockade by fluoxetine did not affect body weight (N=12 for each group, t(22)=1.3, p>0.2).
Blockade or Deletion of SERT did not Affect Pavlovian-to-Instrumental Transfer
We used the PIT to examine the ability of Pavlovian conditioned cues that predict reward to energize operant responding. There was a clear enhancement effect of the Pavlovian cue on responding in both SERT null and WT control mice (light cue effect in nested repeated measures ANOVA, Figure 4a, F(1,21)=16.5, p<0.001), but there was no difference between genotypes (light cue × genotype interaction in nested repeated measures ANOVA, F(1,21)=2.5, p>0.1). Similarly, there was a clear enhancement effect of the Pavlovian cue on responding in both fluoxetine- and saline-treated mice (light cue effect in nested repeated measures ANOVA, Figure 4b, F(1,21)=44.5, p<0.0001), but there was no difference between treatment groups (light cue × treatment interaction in nested repeated measures ANOVA, F(1,21)=0.19, p>0.5).
Neither genetic deletion nor pharmacological blockade of SERT affected Pavlovian-to-Instrumental transfer (PIT). (a) SERT deletion did not affect the ability of a Pavlovian cue to enhance operant responding in PIT. The Pavlovian cue showed significant enhancement effect in both genotypes (F(1,21)=16.5, p<0.001) but there was no genotype difference (N=12 for SERT null, N=11 for WT, F(1,21)=2.5, p>0.1). (b) Fluoxetine treatment had no effect in the PIT task. Ability of a Pavlovian cue to enhance responding was robust (F(1,21)=44.5, p<0.0001), but was insensitive to fluoxetine treatment (N=12 for fluoxetine, N=11 for saline, F(1,21)=0.19, p>0.5).
Blockade or Deletion of SERT Reduced Non-Goal-Directed Responses
In the progressive ratio operant task, we used the inactive control lever to monitor non-goal-directed responses. The absolute level of control lever presses compared to active lever presses was very low in most animals. SERT null mice responded significantly less on the inactive lever in the food-sated (genotype effect in repeated measures ANOVA, Figure 5b, F(1,20)=7.6, p<0.05) condition as well as a trend toward reduced inactive lever responses in the food-deprived condition (genotype effect in repeated measures ANOVA, Figure 5a, F(1,21)=3.4, p=0.08). Similarly, fluoxetine-treated mice responded significantly less on the inactive lever when food-sated (treatment effect in repeated measures ANOVA, Figure 5d, F(1,22)=10.2, p<0.01), and showed a trend toward reduced inactive lever responses when food-deprived (treatment effect in repeated measures ANOVA, Figure 5c, F(1,21)=2.8, p=0.11).
Both genetic deletion and pharmacological blockade of SERT reduced operant responding on inactive levers in the progressive ratio (PR) operant task. (a) SERT-null mice showed a tendency toward a reduction in presses on the inactive lever in the food-deprived condition (N=11 for SERT null group, N=12 for WT group, F(1,21)=3.4, p=0.08). (b) SERT-null mice showed significant reductions in inactive lever presses in the food-sated condition (F(1,20)=7.6, p<0.05). (c) Fluoxetine-treated mice showed a tendency toward a reduction in inactive lever presses in the food-deprived condition (N=11 for fluoxetine group, N=12 for saline group, F(1,21)=2.8, p=0.11). (d) Fluoxetine-treated mice showed a significant reduction in inactive lever presses in the food-sated condition (F(1,22)=10.2, p<0.01).
We further investigated the possibility of activity reduction in SERT-null and fluoxetine-treated mice by using low-lit (25 lux) open field locomotor activity boxes. SERT null mice displayed significantly reduced locomotor activity in both food-deprived (genotype effect in unpaired t-tests Figure 6a, t(21)=3.7, p<0.01) and food-sated (Figure 6b, t(21)=4.5, p<0.001) conditions. Similarly, fluoxetine treatment for 3 weeks' significantly reduced open-field activity as compared to the saline treatment group (treatment effect in unpaired t-test, Figure 6c, t(22)=3.6, p<0.01 for the food-deprived condition and Figure 6d, t(22)=3.5, p<0.01 for the food-sated condition).
Both genetic deletion and pharmacological blockade of SERT reduced locomotor/exploratory activities in the open field. (a) SERT null mice showed significantly reduced total path lengths in the open field in the food-deprived condition (N=11 for SERT null group, N=12 for WT group, t(21)=3.7, p<0.01). (b) SERT null mice showed significantly reduced total path length in the open field in the food-sated condition (t(21)=4.5, p<0.001). (c) Fluoxetine-treated mice showed a significant reduction in total path length in the open field in the food-deprived condition (N=11 for fluoxetine group, N=12 for saline group, t(22)=3.6, p<0.01). (d) Fluoxetine-treated mice showed a significant reduction in total path length in the open field in the food-sated condition (t(22)=3.5, p<0.01).
As locomotor activities in the open field often reflect an animal's reactivity to the novel environment, we examined home cage wheel running activities of SERT null and WT control mice continuously for 2 weeks. There is no significant difference between genotypes in total number of bouts (genotype effect in ANOVA, Figure 7a, F(1,12)=1.4, p>0.2), average revolutions per bout (genotype effect in ANOVA, Figure 7b, F(1,12)=1.4, p>0.2) or average wheel running speed (genotype effect in ANOVA, Figure 7c, F(1,12)=0.67, p>0.4).
SERT deletion did not affect home cage wheel running activities. (a) Total number of bouts in two weeks (N=8 for both genotypes, F(1,12)=1.4, p>0.2). (b) Average revolutions per bout (N=8 for both genotypes, F(1,12)=1.4, p>0.2). (c) Average wheel running speed (N=8 for both genotypes, F(1,12)=0.67, p>0.4).
To examine further whether a motor impairment might contribute to the behavioral phenotypes of SERT null and fluoxetine-treated mice, we analyzed the proportion of progressive ratio responses that had very short inter-response times (IRTs). This analysis did not reveal any differences between SERT null and WT control mice in the ratio of responses with IRTs between 0 and 1 s to total responses (genotype effect in unpaired t-test, Figure 8a, t(20)=1.3, p>0.2 for food deprived and Figure 8b, t(20)=0.90, p>0.3 for food sated conditions). Similarly, fluoxetine-treatment was not associated with any differences in this parameter (treatment effect in unpaired t-test, Figure 8c, t(21)=1.7, p>0.1 for food-deprived and Figure 8d, t(22)=0.72, p>0.4 for food sated conditions).
Neither genetic deletion nor pharmacological blockade of SERT preferentially affected fast operant responses. Fast operant responses were defined as the proportion of responses with inter-response times between 0 and 1 s in the progressive ratio task. (a) SERT null vs WT, food-deprived condition (N=10 for SERT null, N=12 for WT, t(20)=1.3, p>0.2). (b) SERT null vs WT, food-sated condition (N=10 for SERT null, N=12 for WT, t(20)=0.90, p>0.3). (c) Fluoxetine vs saline treatment, food-deprived condition (N=12 per group, t(21)=1.7, p>0.1). (d) Fluoxetine vs saline treatment, food-sated condition (N=12 per group, t(22)=0.72, p>0.4).
DISCUSSION
In the present study, we consistently find that chronic pharmacological blockade and genetic deletion of SERT result in similar behavioral consequences: either manipulation reduces operant responding for natural reward. Reduced operant responding for food reward in mice treated chronically with fluoxetine and in SERT null mice is in line with previous studies reporting declines in responding for drug, alcohol, and intracranial self-stimulation with fluoxetine treatment (Carroll et al, 1990; Hubbell et al, 1991; Richardson and Roberts, 1991; Lee and Kornetsky, 1998; Wilson et al, 2000; Baker et al, 2001; Glatz et al, 2002), suggesting that although previous studies examined non-natural rewards, the effect of fluoxetine can be generalized to different reward types.
The reduction in operant responding after chronic pharmacological blockade or genetic deletion of SERT is not specific for goal-directed responses. To gain additional insight into changes seen with SERT blockade or deletion, it is most relevant to compare these data with the consequences of dopaminergic manipulations. The effects of such manipulations on motivated behaviors have been well characterized. Blockade of dopaminergic receptors leads to reduced operant responding for natural reward. However, such reduction is more specific for goal-directed behavior. In the progressive ratio task utilizing natural reward, dopamine antagonists only reduce operant responding on the active lever, not the inactive one, and only when animals are food deprived (Aberman et al, 1998; Hamill et al, 1999). In the concurrent food choice task, a similar reduction in responding is only seen in the ‘choice’ condition, not the ‘no choice’ condition (Cousins and Salamone, 1994; Cousins et al, 1994, 1996; Salamone et al, 1995, 2001, 2002; Sokolowski and Salamone, 1998; Aberman and Salamone, 1999; Nowend et al, 2001; Correa et al, 2002; Ishiwari et al, 2004; Mingote et al, 2005), suggesting that the role of dopamine becomes important when the task requires the ability to increase effort. Similarly, previous work in our laboratory utilizing genetic repression of dopamine transporter (DAT) expression (which elevates extracellular dopamine levels) has demonstrated increased operant responding for food reward in the progressive ratio task only on the active lever and only when food-deprived (Cagniard et al, 2006). Moreover, increased operant responding for food reward in these mice is seen in the concurrent food choice task only in the ‘choice’ condition, not in the ‘no choice’ condition (Cagniard et al, 2006). In direct contrast, chronic pharmacological blockade or genetic deletion of the SERT reduces operant responding on active as well as inactive levers, it is seen in both food-deprived and food-sated conditions, and it affects operant responding in both ‘choice’ and ‘no choice’ conditions. Moreover, we specifically examined the incentive motivational effect of the Pavlovian conditioned cue to increase operant responding in PIT. It has been reported that nucleus accumbens infusion of amphetamine enhances the incentive motivational effect of the Pavlovian conditioned cue, presumably owing to increases in dopaminergic activity (Wyvell and Berridge, 2000). In contrast, we found that SERT blockade or deletion had no effect in the PIT task, suggesting, first that our SERT manipulation does not affect animals' ability to learn the Pavlovian association, and second, that this manipulation does not specifically affect the incentive motivational properties of the light cue.
A number of empirical studies and computational models have suggested that serotonin and dopamine have opposing functions in motivational processes (Cameron and Williams, 1995; Luciana et al, 1998; Gainetdinov et al, 1999; Daw et al, 2002). Even though elevated serotonergic activity appears to reduce operant responding as elevated dopaminergic activity appears to enhance operant responding for natural rewards, our careful experimental design and data analyses suggest that the motivational opponency view of dopamine and serotonin function is oversimplified. Dopamine and serotonin obviously affect different behavioral processes that modulate motivation in different ways. It is also worth pointing out that in certain paradigms, such as cocaine-induced hyperlocomotion, dopamine and serotonin may even act synergistically (Bubar et al, 2003).
It has been suggested that SERT deletion may result in motor impairment (Holmes et al, 2002b). Our data suggest that SERT null mice have a generalized reduction in motor output or a reduction in response vigor in a novel or challenging environment. In both the open field (a novel environment) and the operant box (in which lever presses may yield potential food reward), SERT null mice have significantly lower responses than the WT control mice. However, in home cage environment, they do not have lower wheel running activities than their WT controls. The lower operant responses of SERT null mice are not owing to reduced fast responses. In the progressive ratio operant task, the proportion of progressive ratio responses that have very short inter-response times did not differ between SERT null and WT control mice.
Another potential confound that could complicate interpretation of our data is appetite. Reduced appetite could potentially explain reduced operant responding for food reward in SERT null and in fluoxetine treated mice. Fluoxetine, like the serotonin releaser fenfluramine, is an appetite suppressant and can cause clinically significant weight loss in humans, although it is not currently used clinically as a weight-loss agent (Halford et al, 2005; Ioannides-Demos et al, 2005). However, in the concurrent choice operant task, neither the total food intake nor the rodent chow intake was significantly affected by SERT deletion or blockade. This contrasts with published studies using the same paradigm and showed the effect of fenfluramine in reducing both lever pressing and chow consumption (Salamone et al, 2002). Moreover, body weight was not affected by either manipulation in our study. Therefore, appetite is unlikely to contribute significantly to the reductions in operant responding we observed.
The present study details for the first time the effects of both chronic SSRI treatment and constitutive SERT deletion on food-reinforced behavior. The fact that pharmacological blockade and genetic deletion of SERT result in similar changes in almost all the parameters that we examined in different behavioral tasks reinforces the utility of the SERT null line for investigation of the mechanisms underlying chronic SSRI treatment, despite the complete loss of SERT activity throughout development and adulthood in SERT null mice. The present study also highlights the importance of understanding the interactions between the serotonin and dopamine systems in both the study of depression and the mechanisms that mediate the therapeutic effects of antidepressants.
References
Aberman JE, Salamone JD (1999). Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92: 545–552.
Aberman JE, Ward SJ, Salamone JD (1998). Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav 61: 341–348.
Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306: 879–881.
Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL (2001). Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berlin) 155: 18–26.
Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A et al (1998). Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (‘Ecstasy’) in serotonin transporter-deficient mice. Mol Pharmacol 53: 649–655.
Bergqvist PB, Bouchard C, Blier P (1999). Effect of long-term administration of antidepressant treatments on serotonin release in brain regions involved in obsessive-compulsive disorder. Biol Psychiatry 45: 164–174.
Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiebot M (1999). Serotonin and tolerance to delay of reward in rats. Psychopharmacology (Berlin) 146: 400–412.
Bubar MJ, McMahon LR, De Deurwaerdere P, Spampinato U, Cunningham KA (2003). Selective serotonin reuptake inhibitors enhance cocaine-induced locomotor activity and dopamine release in the nucleus accumbens. Neuropharmacology 44: 342–353.
Cagniard B, Balsam PD, Brunner D, Zhuang X (2006). Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology 31: 1362–1370.
Cameron DL, Williams JT (1995). Opposing roles for dopamine and serotonin at presynaptic receptors in the ventral tegmental area. Clin Exp Pharmacol Physiol 22: 841–845.
Carroll ME, Lac ST, Asencio M, Kragh R (1990). Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav 35: 237–244.
Correa M, Carlson BB, Wisniecki A, Salamone JD (2002). Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res 137: 179–187.
Cousins MS, Atherton A, Turner L, Salamone JD (1996). Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res 74: 189–197.
Cousins MS, Salamone JD (1994). Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav 49: 85–91.
Cousins MS, Wei W, Salamone JD (1994). Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology (Berlin) 116: 529–537.
Cryan JF, Valentino RJ, Lucki I (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29: 547–569.
Daw ND, Kakade S, Dayan P (2002). Opponent interactions between serotonin and dopamine. Neural Netw 15: 603–616.
Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999). Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283: 397–401.
Glatz AC, Ehrlich M, Bae RS, Clarke MJ, Quinlan PA, Brown EC et al (2002). Inhibition of cocaine self-administration by fluoxetine or D-fenfluramine combined with phentermine. Pharmacol Biochem Behav 71: 197–204.
Halford JC, Harrold JA, Lawton CL, Blundell JE (2005). Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity. Curr Drug Targets 6: 201–213.
Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD (1999). Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav 64: 21–27.
Harrison AA, Liem YT, Markou A (2001). Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology 25: 55–71.
Harrison AA, Markou A (2001). Serotonergic manipulations both potentiate and reduce brain stimulation reward in rats: involvement of serotonin-1A receptors. J Pharmacol Exp Ther 297: 316–325.
Hirschfeld RM (2000). History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 61 (Suppl 6): 4–6.
Hodos W (1961). Progressive ratio as a measure of reward strength. Science 134: 943–944.
Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN (2003c). Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav 2: 365–380.
Holmes A, Murphy DL, Crawley JN (2002a). Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berlin) 161: 160–167.
Holmes A, Murphy DL, Crawley JN (2003a). Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry 54: 953–959.
Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL (2003b). Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology 28: 2077–2088.
Holmes A, Yang RJ, Murphy DL, Crawley JN (2002b). Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology 27: 914–923.
Hubbell CL, Marglin SH, Spitalnic SJ, Abelson ML, Wild KD, Reid LD (1991). Opioidergic, serotonergic, and dopaminergic manipulations and rats' intake of a sweetened alcoholic beverage. Alcohol 8: 355–367.
Ioannides-Demos LL, Proietto J, McNeil JJ (2005). Pharmacotherapy for obesity. Drugs 65: 1391–1418.
Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD (2004). Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res 151: 83–91.
Joel D, Ben-Amir E, Doljansky J, Flaisher S (2004). ‘Compulsive’ lever-pressing in rats is attenuated by the serotonin re-uptake inhibitors paroxetine and fluvoxamine but not by the tricyclic antidepressant desipramine or the anxiolytic diazepam. Behav Pharmacol 15: 241–252.
Lee K, Kornetsky C (1998). Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav 60: 539–544.
Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH et al (2003). Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry 54: 960–971.
Luciana M, Collins PF, Depue RA (1998). Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cereb Cortex 8: 218–226.
Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD (2005). Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci 21: 1749–1757.
Nowend KL, Arizzi M, Carlson BB, Salamone JD (2001). D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav 69: 373–382.
Owens MJ (2004). Selectivity of antidepressants: from the monoamine hypothesis of depression to the SSRI revolution and beyond. J Clin Psychiatry 65 (Suppl 4): 5–10.
Richardson NR, Roberts DC (1991). Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci 49: 833–840.
Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE (2002). Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berlin) 160: 371–380.
Salamone JD, Kurth P, McCullough LD, Sokolowski JD (1995). The effects of nucleus accumbens dopamine depletions on continuously reinforced operant responding: contrasts with the effects of extinction. Pharmacol Biochem Behav 50: 437–443.
Salamone JD, Wisniecki A, Carlson BB, Correa M (2001). Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience 105: 863–870.
Sokolowski JD, Salamone JD (1998). The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav 59: 557–566.
Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R et al (1998). Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA 95: 7699–7704.
Tella SR (1995). Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav 51: 687–692.
Wilson AW, Costall B, Neill JC (2000). Manipulation of operant responding for an ethanol-paired conditioned stimulus in the rat by pharmacological alteration of the serotonergic system. J Psychopharmacol 14: 340–346.
Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004). Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 29: 1331–1343.
Wyvell CL, Berridge KC (2000). Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward ‘wanting’ without enhanced ‘liking’ or response reinforcement. J Neurosci 20: 8122–8130.
Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R (2005). Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods 143: 27–32.
Acknowledgements
This work was supported in part by the NIMH MH66216 (XZ), NIMH MH068542 (RH) and NIDA T32 DA07255-13 (ACS). We thank Barbara Cagniard and Jeff Beeler for helping to set up various behavioral paradigms and Jeff Beeler for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanders, A., Hussain, A., Hen, R. et al. Chronic Blockade or Constitutive Deletion of the Serotonin Transporter Reduces Operant Responding for Food Reward. Neuropsychopharmacol 32, 2321–2329 (2007). https://doi.org/10.1038/sj.npp.1301368
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301368
Keywords
This article is cited by
-
5-HT2C receptor blockade reverses SSRI-associated basal ganglia dysfunction and potentiates therapeutic efficacy
Molecular Psychiatry (2020)
-
Dorsal raphe serotonin neurons inhibit operant responding for reward via inputs to the ventral tegmental area but not the nucleus accumbens: evidence from studies combining optogenetic stimulation and serotonin reuptake inhibition
Neuropsychopharmacology (2019)
-
Serotonin transporter gene promoter methylation status correlates with in vivo prefrontal 5-HTT availability and reward function in human obesity
Translational Psychiatry (2017)
-
Not All Antidepressants Are Created Equal: Differential Effects of Monoamine Uptake Inhibitors on Effort-Related Choice Behavior
Neuropsychopharmacology (2016)
-
Decreased Incentive Motivation Following Knockout or Acute Blockade of the Serotonin Transporter: Role of the 5-HT2C Receptor
Neuropsychopharmacology (2016)