Abstract
The high rate of smoking in schizophrenia may reflect patients' attempts to reduce the side effects of antipsychotic medications, and one mechanism for this reduction may be a reduction in oxidative stress and free radical-mediated brain damage that may contribute to schizophrenic symptoms and to complications of its treatment. Symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS), side effects were assessed with the Simpson and Angus Rating Scale (SAS), and malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) activities were measured in plasma. All of these measures were compared in 130 male inpatients with DSM-IV schizophrenia: 104 smokers and 26 non-smokers. The results showed that the positive PANSS symptoms were lower in smokers than non-smokers (14.5 vs 17.5), while the negative symptoms were lower in those who smoked more cigarettes (r=−0.23). The SAS showed no differences. The CAT activity was correlated with both GSH-Px and SOD activities. Of the three enzymes only the CAT activity was significantly higher in smokers than non-smokers (2.9 vs 1.6 U/ml), but greater SOD activity correlated more cigarettes smoked (r=0.24). Consistent with some protection against oxidative stress, MDA also was significantly lower in smokers than non-smokers (9.2 vs 14.4 nmol/ml). The fewer positive symptoms in smokers and fewer negative symptoms in those who smoked more cigarettes may be a selection bias, but appears to be associated with decreased oxidative stress and lipid peroxidation in schizophrenics who smoke tobacco.
Similar content being viewed by others
INTRODUCTION
Between 40 and 90% of schizophrenic patients smoke, which is substantially higher than a variety of comparison populations (Dalack et al, 1998; Kelly and McCreadie, 1999), including those with other severe mental illnesses (LLerena et al, 2002). These higher rates in schizophrenia persist across cultures and countries and after correcting for possible confounds, such as marital and socio-economic status, alcohol use, antipsychotic use, or institutionalization (de Leon et al, 2002; LLerena et al, 2002; Lohr and Flynn, 1992). Smokers with schizophrenia also smoke more, favor stronger cigarettes and extract more nicotine from their cigarettes (Olincy et al, 1997) than normal smokers. The reasons for this widespread smoking in schizophrenia are not well understood, but they may be attempting to reduce the side effects of antipsychotic medications, to alleviate negative symptoms and/or cognitive deficits associated with schizophrenia (Adler et al, 1998; Kumari and Postma, 2005).
Nicotine in quantities similar to those in cigarette smoke can induce oxidative stress, as shown in vitro and in vivo (Solak et al, 2005). Increased lipid peroxidation occurs in Chinese hamster ovary cells (Yildiz et al, 1998) and pancreatic tissue of rats (Wetscher et al, 1995) that are incubated with nicotine. After intraperitoneal nicotine the liver, lung and heart tissues of rats also show lipid peroxidation (Helen et al, 2003). Furthermore, the plasma of smokers shows increased products of lipid peroxidation (Kharb and Singh, 2000). The significant cognitive, intellectual and behavioral impairments in the offspring of mothers who smoke during pregnancy may be related to oxidative stress and oxidative cellular injury (Ernst et al, 2001).
However, nicotine may also have antioxidant potential and neuroprotective effects. For example, epidemiological data suggest an inverse correlation between nicotine use (in the form of smoking) and the rate of progression of Parkinson's disease (PD) or Alzheimer's disease (AD) (Quik, 2004; Sabbagh et al, 2002). As enhanced oxidative stress contributes to both PD and AD, nicotine might reverse this component of these two diseases (Linert et al, 1999). Experimental data in animal models of PD have also shown the protective effects of nicotine on nigrostriatal degeneration (Soto-Otero et al, 2002).
Recent data suggest that reasonably low dose of nicotine may act as an antioxidant and play an important role for neuroprotective effect, whereas high concentration may induce neurotoxicity and stimulate oxidative stress (Guan et al, 2003).
Patients with schizophrenia may have altered antioxidant enzyme activities and increased levels of lipid peroxidation (Lohr and Browning, 1995; Yao et al, 2001). For example, high levels of superoxide dismutase (SOD), a key enzyme involved in the detoxification of superoxide radicals, have consistently been found in chronic schizophrenic patients (Yao et al, 1998; Zhang et al, 2003). Moreover, high levels of lipid peroxidation products have been reported in plasma (Mahadik et al, 1998), red blood cells (Herken et al, 2001) and cerebrospinal fluid of chronic schizophrenic patients (Lohr et al, 1990). In the current study, we therefore examined SOD and two related antioxidant enzymes: glutathione peroxidase (GSH-Px) and catalase (CAT). Furthermore, we assessed malondialdehyde (MDA) as an indicator of lipid peroxidation. MDA is a key index of membrane pathology, because it is formed from the decomposition of primary and secondary lipid peroxidation products.
Based on the higher smoking rates among schizophrenic patients and the close relationship between nicotine and oxidative stress, as well as the important role of free radicals in the pathophysiology of schizophrenia, we hypothesize that smoking could play a role in the altered antioxidant enzyme activities and levels of lipid peroxidation in schizophrenia. Because smoking is more common among men than women in both normal populations and schizophrenia, as well as gender differences in smoking behaviors, we included only male subjects. Thus, the purpose of this study was to determine (1) any differences in the psychiatric symptoms or in the side effects of antipsychotics in smokers and non-smokers with schizophrenia; (2) any differences in antioxidant enzyme activities and levels of lipid peroxidation in smokers and non-smokers with schizophrenia; (3) whether there were any relationships among altered antioxidant enzyme activities and levels of lipid peroxidation and psychopathological symptoms in smokers and non-smokers.
METHODS
We recruited 130 male schizophrenic inpatients from Beijing Hui-Long-Guan hospital, a Beijing-city-owned psychiatric hospital. All patients were diagnosed on the basis of the Structured Clinical Interview for DSM-IV (SCID). All schizophrenic patients had been receiving stable doses of oral antipsychotic drugs for at least 12 months before entry into the study. All patients were chronic, with at least 5 years of illness, were Han Chinese, and were between 26 and 65 years old.
A complete medical history, physical examination and laboratory tests were obtained from all subjects. Any subjects with physical abnormalities were excluded. All subjects gave written informed consent, which was approved by the institutional Review Board, the Institute of Mental Health, Peking University. In addition, a current cigarette smoking questionnaire was administered to each patient. If the subjects identified themselves as a smoker, then further questions determined the average number of cigarettes per day in 1 week before entry into the study. If the subject was currently a non-smoker, further questions were asked regarding previous smoking behavior including whether or not they had quit smoking. Quitters were excluded from the present study. Based on the questionnaire responses, 104 (80%) were identified as ‘smokers’, whereas 26 (20%) were identified as ‘nonsmokers’. The demographic comparisons of the two groups are shown in Table 1.
Clinical Measures
The patient's psychopathology was assessed on the day of the blood sampling using the Positive and Negative Syndrome Scale (PANSS), which was measured by two psychiatrists who had simultaneously attended a training session in the use of the PANSS before the study began. After training, repeated assessment showed that an inter observer correlation coefficient greater than 0.8 was maintained for the PANSS total score.
The same clinical psychiatrist measured parkinsonian side effects using the Simpson and Angus Rating Scale (SAS) to provide standardized objective assessments of rigidity, tremor, brady-kinesia and salivation. It consists of the following ten items: gait, arm dropping, shoulder shaking, elbow rigidity, wrist rigidity, leg pendulousness, head dropping, glabellar tap, tremor and salivation. Each item is rated on a five-point scale (0–4). All items are assessed using clinical observation and neurological examination. Current treatment with anti-parkinsonian medication was also recorded.
Blood Sampling
Venous blood from the forearm vein was collected between 0700 and 0900 h following an overnight fast. Antioxidant enzymes and lipid peroxidation products were determined by a technician blind to the clinical status of subjects.
Determination of Antioxidant Enzymes and Lipid Peroxidation Products
SOD, GSH-Px and CAT activities were analyzed using established procedures. A full description of the assays has been given in our previous report (Zhang et al, 2006). Briefly, activity of plasma total SOD, GSH-PX or CAT activity was expressed as units per milliliter plasma (U/ml). The inter- and intra-assay coefficients of variation for SOD were 4.1% (n=8) and 3.2% (n=8), for GSH-PX were 4.8% (n=6) and 5.7% (n=6), and for CAT were 4.5% (n=5) and 5.9% (n=5), respectively. Lipid peroxidation levels were monitored by determining the end product of lipid peroxidation MDA using the thiobarbituric acid method. Malondialdehyde results were expressed as nmol/mL.
Statistical Analysis
Demographic characteristics of the smoker and nonsmoker groups were compared using analysis of variance (ANOVA) for continuous variables and chi-squared for categorical variables. As MDA, SOD, GSH-Px and CAT, as well as clinical variables, were all normally distributed in both smoker and nonsmoker groups (Kolmogorov-Smirnov one-sample test), the principal analysis consisted of one-way ANOVA. Because of group differences in age and duration of illness, analysis of covariance was also performed with these covariates. Correlation among biochemical and clinical ratings (eg number of cigarettes smoked) was examined by Pearson correlation coefficients. As both age and education were negatively correlated with cigarettes smoked among all subjects (both p<0.05), a partial correlation was also calculated, with age and education. For the multiple comparisons, we used multivariate regression analysis.
RESULTS
Symptoms and Demographics
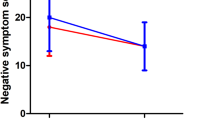
The smoker group scored lower than the nonsmoker group on the positive (14.5±6.3 vs 17.5±4.9) (F=5.17, df=1,128, p<0.002), but not the negative, symptom subscales of the PANSS (24.0±5.9 vs 25.8±6.4; F=0.66; df=1,128; NS). However, a greater number of cigarettes smoked was significantly associated with fewer negative PANSS symptoms (r=−0.23, p=0.02). The SAS total score did not differ between the two groups and was not correlated with the number of cigarettes smoked. Although Table 1 shows significant differences in age (F=7.62, df=1,127, p=0.007) and duration of illness (F=12.4, df=1,127, p=0.001), when both were added as covariates both PANSS symptom associations remained significant.
Antioxidant Enzyme Activities and MDA Levels
The activities of the three enzymes were positively correlated: CAT activity with SOD (r=0.23, p=0.04) and GSH-Px (r=0.24, p=0.02). The CAT activity was significantly higher in smokers than in non-smokers (2.9±0.3 vs 1.6±0.2 U/ml) (F=4.39, df=1,91, p=0.04), and greater SOD activity correlated more cigarettes smoked (r=0.24, df=76, p=0.04), even after covarying for age and education (r=0.27, df=61, p=0.03). Consistent with some protection against rather than response to oxidative stress, MDA was significantly lower in smokers than in non-smokers (9.2±0.7 vs 14.4±1.7 nmol/ml) (F=7.03, df=1,95, p=0.01).
In addition, a four-to-one matching of smokers to non-smokers was carried out thereby minimally reducing the sample size to 24 non-smokers and 96 smokers in a secondary analysis. This eliminated the age and duration of illness difference, but the primary results on the PANSS and biological measures were unchanged (date not shown).
DISCUSSION
We found fewer positive symptoms in schizophrenic smokers than in non-smokers, and that smokers had significantly increased CAT activity and decreased MDA levels. Furthermore, smoking a greater number of cigarettes correlated with fewer negative symptoms and with greater SOD activity. As expected, the activities of the three enzymes were positively correlated.
Clinical data suggest a complex association between smoking and symptoms of schizophrenia (Dalack et al, 1998). For example, some earlier studies such as Goff et al found more positive and negative symptoms in smokers than in non-smokers (Goff et al, 1992), although others such as Ziedonis et al described fewer negative symptoms in heavy smokers compared to light smokers and non-smokers with schizophrenia (Ziedonis et al, 1994). More recent work by Aguilar et al found fewer positive symptoms in smokers than in non-smokers (Aguilar et al, 2005). Furthermore, smoking high-nicotine cigarettes reduced negative symptoms compared to smoking de-nicotinized cigarettes (Smith et al, 2001). Patkar et al (2002) like us also found that smoking was associated with significantly fewer negative symptoms in schizophrenia. Thus, newer studies suggest that smoking may be associated with fewer negative and positive symptoms in schizophrenia.
Our finding of increased CAT activity in smokers and greater SOD activity with greater cigarette consumption is consistent with tobacco smoke leading to oxidative stress and stimulating the protective actions of these enzymes to reverse this stress. In non-schizophrenic smokers, MDA also increases as a product of this oxidative stress (Altuntas et al, 2002; Ozguner et al, 2005; Solak et al, 2005). However, the lower MDA levels in schizophrenic smokers suggests that tobacco smoke increases the activity of these antioxidant enzymes more than is needed to simply counteract the peroxidation effects of tobacco smoke. This excess enzyme activity could offer some protection from lipid peroxidation that otherwise occurs in schizophrenia. Peroxidation damage to dopamine neurons may occur in schizophrenia through subcortical dopamine hyperactivity, because excess catecholamines can auto-oxidize to form free radicals as well as form the toxic dopamine metabolite—6-hydroxydopamine (Lohr and Browning, 1995; Davis et al, 1991).
Nicotine can reduce this peroxidation through several mechanisms and one of these mechanisms also could reduce the positive symptoms of schizophrenia. Nicotine itself reduces lipid peroxidation that is owing to 6-hydroxydopamine autoxidation (Soto-Otero et al, 2002). Furthermore, nicotine can reduce the ability of free radicals to produce 6-hydroxydopamine, which supports an antioxidant mechanism for nicotine's protective effect (Linert et al, 1999). Some component of tobacco smoke also appears to inhibit monoamine oxidase and this also might decrease free radical formation (Mazzio et al, 2005). Furthermore, chronic nicotine administration decreases basal firing rates in the ventral tegmental area dopamine neurons owing to desensitization of the nicotinic receptors (Rasmussen and Czachura, 1995), and chronic nicotine treatment decreases dopamine catabolism in the dorsal striatum (Kirch et al, 1987). Reducing this subcortical dopamine hyperactivity would reduce the positive symptoms in schizophrenia (Davis et al, 1991), as well as decreasing the excess dopamine and the production of 6-hydroxydopamine (Dalack et al, 1998; Davis et al, 1991). Thus, long-term smoking may have several mechanisms that reduce lipid peroxidation in schizophrenia, and like PD, another disease of deteriorating dopamine neurons, tobacco smoking is associated with milder symptoms (Morens et al, 1995).
This study is limited by its sample of chronically hospitalized male patients with more severe psychopathology and longer duration of illness than typical psychotic outpatients or first episode and drug-naïve patients with schizophrenia. Nevertheless, schizophrenic patients who smoke appear to have fewer psychotic symptoms and decreased lipid peroxidation in the context of significantly elevated anti-oxidant enzyme activities. This elevated enzyme activity is consistent with the oxidative stress induced by tobacco smoke, but this stimulated activity appears to overshoot the level needed to reverse the tobacco smoke stress and to possibly reverse a baseline level of ongoing lipid peroxidation associated with schizophrenia itself, chronic antipsychotic medications or some other unidentified cause.
References
Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K et al (1998). Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 24: 189–202.
Aguilar MC, Gurpegui M, Diaz FJ, de Leon J (2005). Nicotine dependence and symptoms in schizophrenia: naturalistic study of complex interactions. Br J Psychiatry 186: 215–221.
Altuntas I, Dane S, Gumustekin K (2002). Effects of cigarette smoking on lipid peroxidation. J Basic Clin Physiol Pharmacol 13: 69–72.
Dalack GW, Healy DJ, Meador-Woodruff JH (1998). Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry 155: 1490–1501.
Davis KL, Kahn RS, Ko G, Davidson M (1991). Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148: 1474–1486.
de Leon J, Becona E, Gurpegui M, Gonzalez-Pinto A, Diaz FJ (2002). The association between high nicotine dependence and severe mental illness may be consistent across countries. J Clin Psychiatry 63: 812–816.
Ernst M, Moolchan ET, Robinson ML (2001). Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adol Psychiat 6: 630–641.
Goff DC, Henderson DC, Amico E (1992). Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiat 149: 1189–1194.
Guan ZZ, Yu WF, Nordberg A (2003). Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem Int 43: 243–249.
Helen A, Krishnakumar K, Vijayammal PL, Augusti KT (2003). A comparative study of antioxidants S-allyl cysteine sulfoxide and vitamin E on the damages induced by nicotine in rats. Pharmacology 67: 113–117.
Herken H, Uz E, Ozyurt H, Sogut S, Virit O, Akyol O (2001). Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol Psychiat 6: 66–73.
Kelly C, McCreadie RG (1999). Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiat 156: 1751–1757.
Kharb S, Singh GP (2000). Effect of smoking on lipid profile, lipid peroxidation and antioxidant status in normal subjects and in patients during and after acute myocardial infarction. Clin Chim Acta 302: 213–219.
Kirch DG, Gerhardt GA, Shelton RC, Freedman R, Wyatt RJ (1987). Effect of chronic nicotine administration on monoamine and monoamine metabolite concentrations in rat brain. Clin Neuropharmacol 10: 376–383.
Kumari V, Postma P (2005). Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev 29: 1021–1034.
Linert W, Bridge MH, Huber M, Bjugstad KB, Grossman S, Arendash GW (1999). In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson's and Alzheimer's diseases. Biochim Biophys Acta 1454: 143–152.
LLerena A, de la Rubia A, Penas-Lledo EM, Diaz FJ, de Leon J (2002). Schizophrenia and tobacco smoking in a Spanish psychiatric hospital. Schizophr Res 58: 323–327.
Lohr JB, Underhill S, Moir S, Jeste DV (1990). Increased indices of free radical activity in the cerebrospinal fluid of patients with tardive dyskinesia. Biol Psychiatry 28: 535–539.
Lohr JB, Flynn K (1992). Smoking and schizophrenia. Schizophr Res 8: 93–102.
Lohr JB, Browning JA (1995). Free radical involvement in neuropsychiatric illnesses. Psychopharmacol Bull 31: 159–165.
Mahadik SP, Mukherjee S, Correnti EE, Scheffer R, Mahadik J (1998). Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol Psychiatry 43: 674–679.
Mazzio EA, Kolta MG, Reams RR, Soliman KF (2005). Inhibitory effects of cigarette smoke on glial inducible nitric oxide synthase and lack of protective properties against oxidative neurotoxins in vitro. Neurotoxicology 26: 49–62.
Morens DM, Grandinetti A, Reed D, White LR, Ross GW (1995). Cigarette smoking and protection from Parkinson's disease: false association or etiologic clue? Neurology 45: 1041–1051.
Olincy A, Young DA, Freedman R (1997). Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 42: 1–5.
Ozguner F, Koyu A, Gokhan C (2005). Active smoking causes oxidative stress and decreases blood melatonin levels. Toxicol Ind Health 21: 21–26.
Patkar AA, Gopalakrishnan R, Lundy A, Leone FT, Certa KM, Weinstein SP (2002). Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis 190: 604–610.
Quik M (2004). Smoking, nicotine and Parkinson's disease. Trends Neurosci 27: 561–568.
Rasmussen K, Czachura JF (1995). Nicotine withdrawal leads to increased firing rates of midbrain dopamine neurons. Neuroreport 7: 329–332.
Sabbagh MN, Lukas RJ, Sparks DL, Reid RT (2002). The nicotinic acetylcholine receptor, smoking, and Alzheimer's disease. J Alzheimers Dis 4: 317–325.
Smith RC, Infante M, Ali A, Nigam S, Kotsaftis A (2001). Effects of cigarette smoking on psychopathology scores in patients with schizophrenia: an experimental study. Subst Abus 22: 175–186.
Solak ZA, Kabaroglu C, Cok G, Parildar Z, Bayindir U, Ozmen D et al (2005). Effect of different levels of cigarette smoking on lipid peroxidation, glutathione enzymes and paraoxonase 1 activity in healthy people. Clin Exp Med 5: 99–105.
Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL (2002). Effects of (−)-nicotine and (−)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson's disease. Biochem Pharmacol 64: 125–135.
Wetscher GJ, Bagchi M, Bagchi D, Perdikis G, Hinder PR, Glaser K et al (1995). Free radical production in nicotine treated pancreatic tissue. Free Radic Biol Med 18: 877–882.
Yao JK, Reddy RD, van Kammen DP (2001). Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs 15: 287–310.
Yao JK, Reddy R, McElhinny LG, van Kammen DP (1998). Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J Psychiatr Res 32: 385–391.
Yildiz D, Ercal N, Armstrong DW (1998). Nicotine enantiomers and oxidative stress. Toxicology 130: 155–165.
Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY (2003). Elevated blood superoxide dismutase in neuroleptic-free schizophrenia: association with positive symptoms. Psychiat Res 117: 85–88.
Zhang XY, Tan YL, Cao LY, Wu GY, Xu Q, Shen Y et al (2006). Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr Res 81: 291–300.
Ziedonis DM, Kosten TR, Glazer WM, Frances RJ (1994). Nicotine dependence and schizophrenia. Hosp Community Psychiat 45: 204–206.
Acknowledgements
This study was funded by the Stanley Medical Institute Foundation (03T-459 and 05T-726) (XYZ), and the Department of Veterans Affairs, VISN 1, Mental Illness Research, Education and Clinical Center (MIRECC) and National Institute on Drug Abuse K05-DA0454 and P50-DA18827 (TRK).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, X., Tan, Y., Zhou, D. et al. Nicotine Dependence, Symptoms and Oxidative Stress in Male Patients with Schizophrenia. Neuropsychopharmacol 32, 2020–2024 (2007). https://doi.org/10.1038/sj.npp.1301317
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301317
Keywords
This article is cited by
-
Variations of plasma oxidative stress levels in male patients with chronic schizophrenia. Correlations with psychopathology and matrix metalloproteinase-9: a case-control study
BMC Psychiatry (2024)
-
Niacin skin flush and membrane polyunsaturated fatty acids in schizophrenia from the acute state to partial remission: a dynamic relationship
Schizophrenia (2022)
-
Demographics, clinical characteristics and cognitive symptoms of heavy smokers and non-heavy smokers in Chinese male patients with chronic schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
The Influence of the Occupational Exposure to Heavy Metals and Tobacco Smoke on the Selected Oxidative Stress Markers in Smelters
Biological Trace Element Research (2014)
-
Psychiatric aspects of chronic lung disease
Current Psychiatry Reports (2009)