Abstract
Long-term pretreatment with an angiotensin II AT1 antagonist blocks angiotensin II effects in brain and peripheral organs and abolishes the sympathoadrenal and hypothalamic–pituitary–adrenal responses to isolation stress. We determined whether AT1 receptors were also important for the stress response of higher regulatory centers. We studied angiotensin II and corticotropin-releasing factor (CRF) receptors and benzodiazepine binding sites in brains of Wistar Hannover rats. Animals were pretreated for 13 days with vehicle or a central and peripheral AT1 antagonist (candesartan, 0.5 mg/kg/day) via osmotic minipumps followed by 24 h of isolation in metabolic cages, or kept grouped throughout the study (grouped controls). In another study, we determined the influence of a similar treatment with candesartan on performance in an elevated plus-maze. AT1 receptor blockade prevented the isolation-induced increase in brain AT1 receptors and decrease in AT2 binding in the locus coeruleus. AT1 receptor antagonism also prevented the increase in tyrosine hydroxylase mRNA in the locus coeruleus. Pretreatment with the AT1 receptor antagonist completely prevented the decrease in cortical CRF1 receptor and benzodiazepine binding produced by isolation stress. In addition, pretreatment with candesartan increased the time spent in and the number of entries to open arms of the elevated plus-maze, measure of decreased anxiety. Our results implicate a modulation of upstream neurotransmission processes regulating cortical CRF1 receptors and the GABAA complex as molecular mechanisms responsible for the anti-anxiety effect of centrally acting AT1 receptor antagonists. We propose that AT1 receptor antagonists can be considered as compounds with possible therapeutic anti-stress and anti-anxiety properties.
Similar content being viewed by others
INTRODUCTION
Angiotensin II (Ang II) contributes to regulate the sympathetic and neuroendocrine systems and it is an important stress hormone (Saavedra, 1992; Phillips, 1997). There are two types of Ang II receptors, the AT1 and AT2 types. The well-known physiological actions of Ang II are dependent on AT1 receptor stimulation; the physiological role of AT2 receptors is controversial (Saavedra, 1999; De Gasparo et al, 2000). AT1 receptors are present throughout the hypothalamic–pituitary–adrenal axis (HPA), highly concentrated in key areas regulating the stress response (Tsutsumi and Saavedra, 1991a, 1991b; Jöhren et al, 1995; Israel et al, 1995). During stress, there is increased renin production and higher circulating and brain Ang II (Xang et al, 1993; Yang et al, 1996), leading to enhanced stimulation of peripheral and brain AT1 receptors. In addition, isolation (Armando et al, 2001; present results) and restraint (Castrén and Saavedra, 1988; Leong et al, 2002; Aguilera et al, 1995a) increased AT1 receptor expression in brain areas inside and outside the blood–brain barrier and related to the control of the hormonal and sympathoadrenal responses to stress, probably as a result of stimulation of glucocorticoid response elements in the receptor promoter region by increased corticosterone levels (Guo et al, 1995). This indicates that stress is likely to increase the effects of brain-generated Ang II and those of circulating Ang II in the brain (Saavedra, 1992).
Stress increases the AT1 receptor expression in the parvocellular hypothalamic paraventricular nucleus (PVN), the site of corticotropin-releasing factor (CRF) formation (Castrén and Saavedra, 1988; Aguilera et al, 1995a; Jezova et al, 1998; Leong et al, 2002), and stimulation of AT1 receptors in the PVN by Ang II increases CRF production (Sumitomo et al, 1991; Aguilera et al, 1995b). AT1 receptors from the PVN are transported to the median eminence through axons coexpressing CRF (Oldfield et al, 2001). Released into the hypothalamic portal system, CRF increases pituitary ACTH. These findings indicate that upregulation of AT1 receptors in the PVN is a major factor modulating the increased CRF production, which is followed by a cascade of stimulated ACTH release and increased adrenal corticoid secretion, the hallmark of the stress reaction.
Because AT1 receptor stimulation enhanced CRF formation and release during stress, it was reasonable to ask whether a limitation of the Ang II tone maintained over time, such as that resulting from long-term antagonism of AT1 receptors, could decrease or prevent the hormonal response to stress. We demonstrated that long-term treatment with candesartan, an insurmountable AT1 antagonist that, when administered peripherally, readily inhibits not only peripheral but also central AT1 receptors (Nishimura et al, 2000), abolished the HPA axis and sympathoadrenal response to isolation in rats (Armando et al, 2001). Isolation is a clinically relevant model of emotional stress resulting from the restriction from freely regulating exposure to novel surroundings and access to familiar territory. In addition, candesartan pretreatment prevented the gastric ulceration produced by cold-restraint stress in rats (Bregonzio et al, 2003). This suggested that antagonism of peripheral and brain AT1 receptors could be of therapeutic relevance in the control of the stress reaction (Armando et al, 2001).
In addition to the hypothalamus, brain AT1 receptors are expressed in many other areas including the cortex, indicating the possibility of a role of Ang II in behavior (Tsutsumi and Saavedra, 1991a; Lenkei et al, 1998). Overexpression of AT1 receptors in mice lacking AT2 receptors (Armando et al, 2002) associates with anxiety-like behavior (Okuyama et al, 1999). Of particular interest was the finding that peripheral administration of the AT1 receptor antagonist losartan reduces anxiety in rodents (Barnes et al, 1990). These findings suggest that AT1 receptor stimulation enhances anxiety and that these receptors regulate not only the autonomic and hormonal but also the behavioral response to stress.
We asked the question whether AT1 receptor antagonists could modulate the response of cortical and subcortical structures to stress. We focused on systems that play recognized roles in stress and anxiety, the cortical, amygdaloid, and septal CRF receptors, cortical benzodiazepine binding sites (part of the inhibitory GABAA complex) (Nutt and Malizia, 2001; Zavala, 1997; Biggio et al, 1990), and tyrosine hydroxylase (TH) in the locus coeruleus, the site of origin of noradrenergic neurons innervating the cortex (Koob, 1999; Dunn and Berridge, 1990; Whitnall, 1993). We tested the effects of long-term pretreatment with an AT1 antagonist on cortical and subcortical CRF and cortical benzodiazepine receptor binding and TH mRNA in the locus coeruleus in animals subjected to isolation stress, and studied the effect of a similar treatment on the behavior of the animals in the elevated plus-maze, a conflict test reflecting anxiety (Lister, 1987).
MATERIALS AND METHODS
Animals and Preparation of Tissues
Wistar Hannover male rats (8 weeks old) were purchased from Taconic Farms, Germantown, NY, kept at 22°C under a 12 : 12-h dark–light cycle with lights on at 0700 hours and were given free access to normal rat diet and tap water. The NIMH Animal Care and Use Committee approved all procedures. All efforts were made to minimize the number of animals used and their suffering (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 80-23, revised 1996).
We used different groups of six rats each to determine (a) Ang II receptor type binding and TH mRNA, (b) CRF receptor and benzodiazepine binding, and (c) behavior in the elevated plus-maze.
Experiment 1. Determination of Ang II Receptor Binding and TH mRNA
Rats were anesthetized with pentobarbital (30 mg/kg), and Alzet osmotic minipumps (Alza Scientific Products, Palo Alto, CA) were implanted subcutaneously. Groups of animals received minipumps containing vehicle or candesartan (ASTRA, Mölndal, Sweden) dissolved in 1 mol/l sodium carbonate and further diluted in isotonic saline, at a final pH of 7.5–8.0, to be delivered at a rate of 0.5 mg/kg/day. The dose of 0.5 mg/kg/day was selected because it produced a very significant decrease in binding to brain AT1 receptors (Nishimura et al, 2000) and was effective in blocking the sympathoadrenal and hormonal response to isolation stress (Armando et al, 2001). After minipump implantation, the rats were kept in their cages in groups of 3–4 for 13 days.
For the isolation experiments, at the end of day 13 of treatment, animals treated with candesartan or vehicle were individually housed in standard, 50 square inch plastic metabolic cages (Nalgene, Rochester, NY) that were located in the same animal room. Control animals (referred as grouped rats) treated with candesartan 0.5 mg/kg/day or vehicle remained grouped 3–4 animals per cage and undisturbed in the same animal room as the isolated rats. Regular rat food and water were provided ad libitum throughout the experiment. At the end of the experiment, on day 14, all animals were killed by decapitation and the brains were removed, frozen in isopentane at −30°C on dry ice, and stored at −80°C until used. These animals were used to determine Ang II receptor binding and TH mRNA as described below.
Experiment 2. Autoradiographic Determination of CRF Receptor and Benzodiazepine Binding
Additional groups of 8-week-old Wistar Hannover rats were housed, treated as above with vehicle or candesartan for 13 days, submitted to isolation stress, killed at the end of day 14, and the brains were removed and processed as described above. These animals were used to determine CRF and benzodiazepine receptor binding as described below.
Experiment 3. Study on the Elevated Plus-Maze
Additional groups of 8-week-old Wistar Hannover rats were housed in groups of three to four rats and treated as above with vehicle or candesartan for 13 days. On day 14, between 0900 and 1100, the animals were tested in the elevated plus-maze as described below.
Ang II Receptor Binding
We cut 16-μm-thick brain coronal sections in a cryostat at −20°C, thaw-mounted the sections on poly-1-lysine-coated slides (Labscientific Inc., Livingston, NJ), dried them overnight in a desiccator at 4°C, and stored them at −80°C until used. Sections were labeled in vitro with 0.5 nM of [125I]Sarcosine1-Ang II ([125I]Sar1-Ang II, Peninsula Laboratories, Belmont, CA; iodinated by the Peptide Radioiodination Service Center, School of Pharmacy, University of Mississippi, to a specific activity of 2176 Ci/mmol). Sections were preincubated for 15 min at 22°C in 10 mM Na phosphate buffer, pH 7.4, containing 120 mM NaCl, 5 mM Na2EDTA, 0.005% bacitracin (Sigma Chemical, St Louis, MO), and 0.2% bovine serum albumin proteinase free (Sigma Chemical), followed by incubation for 120 min in fresh buffer containing 0.5 nM of [125I]Sar1-Ang II. We determined total binding by incubating the sections as described above (Tsutsumi and Saavedra, 1991a). Nonspecific binding was determined in consecutive sections incubated as above in the presence of 1 μM unlabeled Ang II (Peninsula), and was the binding remaining in the presence of excess unlabeled agonist. Specific binding to all Ang II receptors was the difference between total binding and nonspecific binding, which is the binding displaced by excess labeled agonist. To determine selective binding to the Ang II receptor types (AT1 and AT2 receptors), we incubated consecutive sections with 0.5 nM of [125I]Sar1-Ang II in the presence of concentrations of the selective AT1 receptor antagonist losartan (10 μM; DuPont-Merck, Wilmington, DE, USA) or the selective AT2 receptor antagonist PD 123319 (1 μM; Sigma), selected to give maximum specific displacement. The number of AT1 and AT2 receptors was the binding displaced by the AT1 or AT2 receptor antagonists, respectively (Tsutsumi and Saavedra, 1991a).
After incubation, slides were rinsed four consecutive times, for 1 min each, in fresh ice-cold 50 mM Tris-(hydroxymethyl)aminomethane.HCl buffer, pH 7.6, followed by a dip in ice-cold distilled water, and the sections were dried under air (Tsutsumi and Saavedra, 1991a). Sections were exposed to Kodak Biomax MR film (Eastman Kodak Company, Rochester, NY) together with 14C-labeled microscales (American Radiolabeled Chemicals, St Louis, MO). Films were developed in ice-cold GBX developer (Eastman Kodak) for 4 min, fixed in Kodak GBX fixer for 4 min at 22°C, and rinsed in water for 15 min. Optical densities of autoradiograms generated by incubation with the 125I-labeled ligands were normalized after comparison with 14C-labeled standards as described (Miller and Zahniser, 1987), by computerized densitometry using the Image 1.6 Program (National Institute of Mental Health, Bethesda, MD). The films were exposed for different times, depending on the amount of binding present, to obtain film images within the linear portion of the standard curve and transformed to corresponding values of fmol/mg protein (Nazarali et al, 1989; Miller and Zahniser, 1987). Because we used single ligand concentrations below saturation, there is no information as to whether the changes described represent alterations in receptor number or receptor affinity. Each animal was quantified independently. Brain regions were identified according to Paxinos and Watson (1986) by staining of consecutive sections with toluidine blue.
In Situ Hybridization of TH mRNA
For in situ hybridization experiments, 16-μm-thick brain sections consecutive to those used for receptor binding were collected as mentioned above and stored at −80°C until assayed. We synthesized one antisense oligonucleotide of 48-mer for the rat TH cDNA sequence (Lofstrand Labs Limited, MD), localized in nt 1562–1609 (Grima et al, 1985), and labeled the oligonucleotide with terminal deoxynucleotidyl transferase (Amersham) to a specific activity of 3–4 × 108 dpm/μg. Each reaction was performed with 70 pmol of oligonucleotides in the presence of 70 μCi of [α-35S]ATP (Amersham). The labeled oligonucleotides were separated from unincorporated nucleotides using MicroSpin G-25 columns (Amersham). In situ hybridization of rat brain sections and posthybridization washings were performed as described (Wisden and Morris, 1994) in consecutive brain sections, one incubated with labeled antisense oligonucleotide and another with labeled oligonucleotide in the presence of excess unlabeled probe (157 pmol/ml). After exposure to BioMax MR films (Kodak), the films were developed and quantified by comparison with 14C-labeled standards (American Radiolabeled Chemicals).
Autoradiography of CRF Receptors
We cut 16-μm-thick brain coronal sections in a cryostat at −20°C, thaw-mounted the sections on poly-1-lysine-coated slides (Labscientific Inc., Livingston, NJ), dried them overnight in a desiccator at 4°C, and stored them at −80°C until used.
Consecutive brain sections were preincubated twice for 10 min in 50 mM Tris buffer, pH 7.4, followed by incubation for 60 min at room temperature in 50 mM Tris buffer, pH 7.4, containing 10 mM MgCl2, 0.1% BSA, 0.05% bacitracin, and 0.2 nM [125I]sauvagine (specific activity 2200 Ci/mmol; Perkin-Elmer, Boston, MA) to label both CRF1 and CRF2 receptor subtypes (Rominger et al, 1998). Consecutive sections were used to determine selective binding to CRF1 and CRF2 receptors. Binding to both CRF1 and CRF2 receptors was calculated as the binding of [125I]sauvagine displaced by 1 μM human CRF (Peninsula). The binding not displaced by 1 μM human CRF was defined as nonspecific binding. Binding to CRF1 receptors was the [125I]sauvagine binding displaced by 13 nM of the selective CRF1 receptor antagonist antalarmin (Rominger et al, 1998; Schulz et al, 1996; Webster et al, 1996; McCarthy et al, 1999). The [125I]sauvagine binding not displaced by antalarmin but displaced in the presence of 1 μM hCRF was considered as binding to CRF2 receptors. Following the incubation period, slides were washed twice, 5 min each, in Tris buffer (50 mM) containing 0.01% Triton X-100 at 4°C. Slides were washed in deionized water, dried under cold air, exposed to Kodak Biomax MR film (Eastman Kodak Company, Rochester, NY) together with 14C-labeled standards, and developed and quantified as described above. Cortical areas (cingulate, frontal, and parietal), the lateral septal nucleus, and the amygdaloid complex were defined according to Paxinos and Watson (1986).
Autoradiography of Benzodiazepine Binding Sites
Brain sections (16-μm-thick) were incubated for 90 min at 4°C in assay buffer (50 mM Tris-citrate pH 7.1 containing 150 mM NaCl) and 1 nM of the nonselective benzodiazepine agonist [3H]flunitrazepam (71.0 Ci/mmol; Perkin Elmer, Boston, MA). The binding of [3H]flunitrazepam displaced in the presence of 1 μM clonazepam in consecutive sections was considered as binding to central benzodiazepine receptors (BZ1 and BZ2) (Negro et al, 1995; Fernández-López et al, 1997). After incubation, sections were washed five times for 5 min in incubation buffer at 4°C and dipped once in ice-cold distilled water. Slides were dried and exposed to Kodak Biomax MR film for 1 week together with 3H-labeled standards. Films were developed as above, and images quantified as described above with comparison to 3H-labeled standards (Orchinik et al, 2001). Cortical areas were defined as above (Paxinos and Watson, 1986).
Elevated Plus-Maze
The plus-maze apparatus was made of stainless steel and consisted of four arms elevated 50 cm above the ground, with each arm (50 cm long and 10 cm wide) positioned 90° relative to the adjacent. The arms extended from a central platform with two closed arms (walls 40 cm high) and two open arms (Columbus Instruments, Columbus, OH). Testing was conducted in a quiet room. To facilitate adaptation, the animals were placed in the behavioral room 1 h before testing.
Rats were placed in the center of the plus-maze facing an open arm (Rodgers and Johnson, 1998), and we recorded the percent of time spent in the open arms and the number of entries in the open and closed arms. Arm entry was defined as placing all four paws on it, and the duration of the test was 5 min for each animal (Montgomery, 1955).
Statistics
Data are means±SEM, for groups of six animals measured individually. Two-way ANOVA followed by the Newman–Keuls test was used to assess the significance of differences in receptor binding, TH mRNA, and CRF content among groups. Unpaired Student's t-test was used to assess the significance of differences in the behavior display in the plus-maze. p<0.05 was considered as statistically significant.
RESULTS
Effect of Isolation and AT1 Antagonism on Expression of Ang II Receptors and TH mRNA in the Brain
In grouped animals, subcutaneous administration of the AT1 antagonist for 14 days substantially decreased the binding to AT1 receptors in all areas studied. The binding to AT2 receptors was not affected by the treatment (Table 1). The significant reduction in binding to AT1 receptors in grouped, nonstressed animals very likely represents insurmountable binding of candesartan (Nishimura et al, 2000; Armando et al, 2001).
In vehicle-treated animals, isolation increased significantly AT1 binding in the PVN, subfornical organ, nucleus of the solitary tract, and area postrema (Table 1). Conversely, isolation significantly decreased the binding to AT2 receptors in the locus coeruleus and inferior olive (Table 1 and Figure 1). Pretreatment of the animals with the AT1 antagonist abolished the increase in AT1 receptors in all areas studied and reversed the decrease in AT2 binding in the locus coeruleus and the inferior olive (Table 1 and Figure 1).
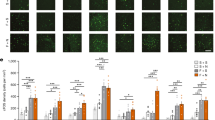
Quantification of AT2 receptors and of in situ hybridization of TH mRNA in the locus coeruleus. Grouped or isolated rats were treated for 14 days with vehicle or the AT1 receptor antagonist. Values are means±SEM for groups of six rats, measured individually as described under Materials and methods. *p<0.05 as compared to all others.
Administration of the AT1 antagonist to grouped animals had no effect on the expression of TH mRNA in the locus coeruleus. Isolation significantly increased TH mRNA in vehicle-treated animals, and pretreatment with the AT1 antagonist completely prevented the isolation-induced increase in TH mRNA (Figure 1).
Effect of Isolation and AT1 Receptor Antagonism on Expression of CRF1 Receptors in Brain Cortex
Addition of unlabeled CRF completely displaced cortical binding of [125I]sauvagine (Figure 2). In the cortex, most of the [125I]sauvagine binding was displaced by antalarmin, indicating a predominance of CRF1 receptors (Figure 2). CRF1 binding was unevenly distributed in the parietal cortex, with layer IV, corresponding to the granular layer, expressing about two-fold higher binding than cortical layers I–III and V–VI (Figures 2, 3 and 4).
Autoradiography of CRF receptor types in the rat cortex. Upper figure: Autoradiographic images of cortical sections incubated in the presence of 0.2 nM of [125I]sauvagine to reveal CRF receptors. Middle figure: Consecutive section incubated as above with addition of antalarmin to displace binding to CRF1 receptors. Lower figure: Consecutive section incubated as above with addition of unlabeled CRF to displace binding to CRF1 and CRF2 receptors (see Materials and methods).
Representative autoradiography of CRF receptor binding in cortex. Grouped or isolated rats were treated for 14 days with vehicle or the AT1 antagonist. Sections were incubated with [125I]sauvagine as described in Materials and methods and represent total binding. Note that the decreased cortical binding in isolated animals treated with vehicle was prevented by pretreatment with the AT1 receptor antagonist.
Quantification of CRF1 receptors in the cingulate, frontal, and parietal cortex. Grouped or isolated animals were treated with vehicle or the AT1 antagonist. Values are means±SEM for groups of six rats, measured individually as described under Materials and methods, and are expressed as fmol/mg protein. *p<0.05 as compared to all other experimental groups.
The number of cortical CRF2 receptors (binding not displaced by antalarmin but displaced by unlabeled CRF) represented about 25–40% of the total binding to CRF receptors (Table 2 and Figure 2). Higher numbers of CRF2 receptors were expressed in layer IV of the parietal cortex (Table 2).
Pretreatment of grouped animals with the AT1 antagonist had no effect on the binding of [125I]sauvagine to CRF1 receptors in any of the brain cortical areas examined (Figure 4). Isolation significantly decreased CRF1 receptor binding, about 35–40%, in all cortical layers examined (Figures 3 and 4). In all cortical layers, pretreatment with the AT1 antagonist completely prevented the decrease in CRF1 binding, which occurred in animals subjected to isolation stress (Figures 3 and 4).
Conversely, the expression of CRF2 receptors was not significantly altered by isolation or pretreatment with the AT1 receptor antagonist, in any of the cortical areas studied (Table 2).
Effect of Isolation and AT1 Receptor Antagonism on Expression of CRF Receptors in Septum and Amygdala
In the lateral septum, only CRF2, but not CRF1, receptors were expressed. There was no significant change in CRF2 receptors when grouped animals were treated with candesartan, and no significant changes in CRF2 receptor expression were detected after isolation. Pretreatment of isolated animals with candesartan produced a small (15%) but statistically significant increase in CRF2 receptor expression. Values were 2.38±0.20, 2.08±0.25, 2.19±0.15, and 2.53±0.12 fmol/mg protein for grouped, grouped treated with candesartan, isolated, and isolated treated with candesartan, respectively (p<0.05, isolated treated with candesartan vs all other groups).
In the amygdala complex, we detected both CRF1 and CRF2 receptors. There were no significant changes in expression of either receptor type after treatment of grouped animals with candesartan, after isolation, or after pretreating isolated animals with candesartan. Values for CRF1 receptors were 1.43±0.20, 0.90±0.38, 1.47±0.23, and 0.99±0.21 fmol/mg protein, respectively, for grouped, grouped treated with candesartan, isolated, and isolated pretreated with candesartan (p>0.05). Values for CRF2 receptors were 1.86±0.17, 1.97±0.18, 1.41±0.20, and 1.77±0.20 fmol/mg protein, respectively, for grouped, grouped treated with candesartan, isolated, and isolated pretreated with candesartan (p>0.05).
Effect of Isolation and AT1 Receptor Antagonism on Expression of Central Benzodiazepine Binding Sites in Brain Cortex
The binding of [3H]flunitrazepam to cortical areas was completely displaced by 1 μM clonazepam, indicating binding to the central type benzodiazepine BZ1 and BZ2 receptors (Figure 5).
Autoradiography of benzodiazepine binding in cortex. Upper figure: Autoradiographic images of cortical sections incubated in the presence of 1 nM of [3H]flunitrazepam. Lower figure: Consecutive section incubated as above with addition of clonazepam to displace binding to benzodiazepine sites (see Materials and methods).
Benzodiazepine binding was unevenly distributed in the cortical areas studied. Highest binding was present in the cingulate cortex and in layer IV, corresponding to the granular layer, of the parietal cortex (Figures 5, 6 and 7).
Representative autoradiography of benzodiazepine binding in cortex. Grouped or isolated rats were treated for 14 days with vehicle or the AT1 antagonist. Sections were incubated with [3H]flunitrazepam as described in Materials and methods. Note that the decreased cortical binding in isolated animals treated with vehicle was prevented by pretreatment with the AT1 receptor antagonist.
Quantification of benzodiazepine receptors in the cingulate, frontal, and parietal cortex. Grouped or isolated animals were treated with vehicle or the AT1 antagonist. Values are means±SEM for groups of six rats, measured individually as described under Materials and methods, and are expressed as fmol/mg protein. *p<0.05 as compared to all other experimental groups.
Pretreatment of the animals with the AT1 receptor antagonist did not modify the binding to central benzodiazepine receptors in grouped animals (Figures 6 and 7). In animals subjected to isolation stress, benzodiazepine binding was significantly decreased in all cortical areas studied (Figures 6 and 7), and this decrease was completely prevented by pretreatment of the animals with the AT1 receptor antagonist (Figures 6 and 7).
Effect of Pretreatment with an AT1 Receptor Antagonist on the Behavior in the Elevated Plus-Maze
Administration of the AT1 antagonist to grouped animals for 13 days before the testing increased the number of entries into open arms and increased the percent of the time spent in the open arms. Entries into closed arms were not affected by the treatment (Figure 8).
Behavior in the elevated plus-maze. Grouped undisturbed rats pretreated for 14 days with vehicle or the AT1 antagonist were tested in the elevated plus-maze and measured individually as described under Materials and methods. Values are means±SEM for groups of 10 rats. *p<0.05 as compared to the vehicle-treated group.
DISCUSSION
The main finding of this study is that pretreatment with a centrally acting Ang II AT1 receptor antagonist prevents the isolation stress-induced alterations in cortical CRF1 and benzodiazepine binding and locus coeruleus TH mRNA, and reduces anxiety in the elevated plus-maze. This indicates that AT1 receptor antagonists exert anti-stress and anti-anxiety properties by modulating three interacting cortical systems, CRF, GABAA, and norepinephrine.
We confirmed that subcutaneous administration of the insurmountable and selective AT1 receptor antagonist candesartan blocked brain AT1 receptors, demonstrating that the compound crossed the blood–brain barrier and is an effective agent to antagonize the effects of brain Ang II (Nishimura et al, 2000; Seltzer et al, 2004). Pretreatment with the AT1 antagonist, by preventing the hormonal response to isolation, prevented the glucocorticoid-induced increase in receptor transcription (Armando et al, 2001; Leong et al, 2002; present results) and the corresponding increase in expression of AT1 receptors in the PVN (Armando et al, 2001).
The locus coeruleus, the site of origin of the sympathetic innervation to the cortex, participates in the well-characterized stress-induced central sympathetic stimulation (Carrasco and Van de Kar, 2003; Berridge and Waterhouse, 2003). AT1 stimulation enhances central norepinephrine formation and release (Saavedra, 1992). Pretreatment with AT1 antagonists prevented the sympathoadrenal response to isolation (Armando et al, 2001) and the increase in TH mRNA in the locus coeruleus after central administration of Ang II (Seltzer et al, 2004). For these reasons, it was not surprising to find that pretreatment with candesartan prevented the stress-induced increase in TH mRNA in the locus coeruleus (present results).
However, in the rat, the locus coeruleus does not express AT1 receptors, but large numbers of Ang II AT2 receptors (Tsutsumi and Saavedra, 1991a; present results). Isolation (present results) or cold stress (Peng and Phillips, 2001) decrease AT2 binding in the locus coeruleus, a change in opposite direction to that of forebrain and brainstem AT1 receptors during stress. We found that, in parallel with a reversal of the isolation-induced increase in TH mRNA, candesartan prevented the isolation-induced decrease in AT2 binding in the locus coeruleus. These findings suggest that, whereas brain AT1 receptors are clearly involved in the control of the central sympathetic drive through regulation of TH transcription, AT1 receptor antagonists prevent the stress-induced increase in central sympathetic drive by indirect effects requiring AT2 receptor participation. In support of this hypothesis, we reported a dual role for AT1 and AT2 receptors in the control of basal TH transcription and catecholamine formation in the adrenal medulla (Jezova et al, 2003).
The coordination of behavioral and autonomic responses to stress, including fear and anxiety (Dunn and Berridge, 1990; Whitnall, 1993; Carrasco and Van de Kar, 2003), is partially under the control of extrahypothalamic, including cortical, CRF neurons predominantly expressing CRF1 receptors (Bittencourt and Sawchenko, 2000; Chalmers et al, 1995; Van Pett et al, 2000). CRF1 receptor activity is important for the induction of anxiety, and CRF1 (corticotropin-releasing hormone) receptor antagonists decrease stress-induced anxiety (Menzaghi et al, 1994; Rodriguez de Fonseca et al, 1996; Millan et al, 2001; Smith et al, 1998).
Cortical CRF1 receptor binding decreases after isolation (present results), foot shock (Anderson et al, 1993), and chronic unpredictable stress (Iredale et al, 1996). Central administration of CRF downregulated CRF1 binding in the frontal cortex (Brunson et al, 2002) and incubation of a neuron-derived cell line with CRF decreased the levels of CRF1 mRNA (Iredale et al, 1996). For these reasons, the stress-induced decrease in CRF1 receptors has been related to ligand-induced downregulation in response to increased peptide release (Carrasco and Van de Kar, 2003). In support of this hypothesis, we found a decrease in cortical CRF levels of rats submitted to cold restraint, a change prevented by pretreatment with candesartan (unpublished results).
We report that pretreatment with the AT1 antagonist candesartan prevents the isolation-induced decrease in cortical CRF1 binding. The stress-induced release of cortical CRF may be positively regulated by cortical AT1 receptor stimulation, in a manner similar to that occurring at the hypothalamic level. Autoradiographic studies revealed AT1 receptors in the entorhinal and piriform cortex, but not in the neocortex (Tsutsumi and Saavedra, 1991a), possibly because of limitations in the power of cellular resolution of the film autoradiography. However, expression on neocortical AT1 receptor mRNA was detected with in situ hybridization (Lenkei et al, 1998), indicating the existence of a cortical AT1 receptor system. Thus, blockade of cortical AT1 receptors could directly reduce CRF release and prevent CRF1 receptor downregulation. Alternatively, or in addition, AT1 receptor antagonism could prevent the stress-induced decrease on cortical CRF1 receptors by decreasing TH transcription in the locus coeruleus. There is a reciprocal relationship between the brain CRF and sympathetic systems, and CRH contributes to activation of the locus coeruleus during stress (Berridge and Waterhouse, 2003). Stress increases CRF concentrations in the locus coeruleus (Chappell et al, 1986), local application of CRF in the locus coeruleus induces behavioral activation (Butler et al, 1990), and i.c.v. administration of a CRF antagonist blunts the stress-induced increase in extracellular norepinephrine levels in the prefrontal cortex (Shimizu et al, 1994). This in turn could decrease CRF release from cortical neurons, as it is known that at least in the hypothalamus CRF release is under noradrenergic control (Szafarczyk et al, 1995).
In addition to CRF1 receptors, there are cortical CRF2 receptors in rats (Primus et al, 1997) and nonhuman primates (Sánchez et al, 1999). The modulatory effect of the AT1 receptor antagonist appears restricted, in cortical areas, to CRF1 receptors, as the expression of cortical CRF2 receptors is not altered by candesartan pretreatment.
The role of brain AT1 receptors may not be limited to that of regulatory functions in cortical structures, the focus of the present study, but may very well extend to subcortical limbic structures such as the amygdala, septum, and hippocampus, the site of large numbers of AT1 receptors (Tsutsumi and Saavedra, 1991a). For this reason, we examined the effects of isolation and candesartan treatment on the expression of CRF receptors in the septum and amygdaloid complex, part of a circuit that plays a major role in the regulation of the stress response (Carrasco and Van de Kar, 2003; Herman et al, 2003). In the amygdaloid complex, we found no isolation-induced alterations and no changes after candesartan treatment in CRF1 or CRF2 receptor expression, indicating that the effects of AT1 receptor blockade in CRF1 receptors may be restricted to cortical areas.
In our experiments, we did not detect significant numbers of CRF1 receptors in the lateral septum, a region with very low expression of CRF1 receptor mRNA (Chalmers et al, 1995). In isolated rats treated with candesartan, there was a small increase in septal CRF2 receptors. This finding may be of interest because activation of CRF2 receptors reverses anxiety-like behavior (Valdez et al, 2004) and CRF2 receptors have been proposed as regulators of the stress response (Risbrough et al, 2004).
In the cortex, CRF negatively modulates the activity of the GABAA complex, the main central inhibitory system (Takamatsu et al, 1991; Serra et al, 1999). The CRF and GABAA systems are tightly interconnected, and in the PVN, GABAA receptors colocalize with CRF neurons (Cullinan, 2000). A similar interaction is likely to occur in the cortex. The effect of CRF1 antagonists is similar to the effect of the benzodiazepines, the classical anxiolytic compounds, which stimulate central benzodiazepine sites, part of the inhibitory GABAA receptor complex (Nutt and Malizia, 2001; Zavala, 1997; Biggio et al, 1990). Stimulation of central benzodiazepine receptors increases the affinity of GABA for its binding site through positive allosteric effects, potentiating GABAergic transmission (Zavala, 1997). Isolation (present results) or exposure to inescapable stressors such as foot shock or forced swimming (Lippa et al, 1978; Weizman et al, 1989; Medina et al, 1983) decreased benzodiazepine receptor binding in the frontal cortex. In turn, decreased benzodiazepine binding decreases GABAergic transmission, and this leads to stress-induced anxiety (Nutt and Malizia, 2001). Our finding of decreased cortical benzodiazepine receptor binding during isolation is most likely associated with the stress-induced increase in cortical CRH release. By decreasing CRF release, AT1 receptor blockade would also reverse the stress-induced decrease in central benzodiazepine binding and restore the inhibitory influence of the GABAA complex during isolation.
In the elevated plus-maze, a test of anxiety-related behavior (Lister, 1987), pretreatment with candesartan increased the number of entries into the open arm of the maze and the time spent in the open arm, indicating a clear anxiolytic effect, similar to that found after peripheral administration of other AT1 receptor antagonists (Barnes et al, 1990; Kaiser et al, 1992) and to that of CRF1 receptor antagonists (Korte and De Boer, 2003; Millan et al, 2001).
Our results are not without clinical implications. Hyperactivity of the HPA axis and of CRF neurons regulating higher brain centers are confirmed findings in anxiety and in stress-related affective disorders (Bremner et al, 2000; Keck and Holsboer, 2001). We demonstrate here that inhibition of Ang II AT1 receptors is sufficient to block stress-induced changes in CRF1 receptors and to restore the inhibitory effect of the cortical GABAA system. Our hypothesis is that these effects explain the anxiolytic and anti-stress effects of centrally active AT1 receptor antagonists.
Our observations indicate that Ang II AT1 receptors are involved in higher regulatory mechanisms controlling the behavioral and cognitive responses to stress and anxiety. Antagonism of brain Ang II AT1 receptors could open a new lead in the treatment of anxiety and other stress-related psychiatric conditions such as depression and post-traumatic stress disorder.
References
Aguilera G, Kiss A, Luo X (1995a). Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J Neuroendocrinol 7: 775–783.
Aguilera G, Young WS, Kiss A, Bathia A (1995b). Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology 61: 437–444.
Anderson SM, Kant GJ, De Souza EB (1993). Effects of chronic stress on anterior pituitary and brain corticotropin-releasing factor receptors. Pharmacol Biochem Behav 44: 755–761.
Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA et al (2001). Peripheral administration of an angiotensin II AT1 receptor antagonist decreases the hypothalamic–pituitary–adrenal response to stress. Endocrinology 142: 3880–3889.
Armando I, Terrón JA, Falcón-Neri A, Ito T, Häuser W, Inagami T et al (2002). Increased angiotensin II AT1 receptor expression in paraventricular nucleus and hypothalamic–pituitary–adrenal axis stimulation in AT2 receptor gene-disrupted mice. Neuroendocrinology 76: 137–147.
Barnes NM, Costall B, Kelly ME, Murphy DA, Naylor RJ (1990). Anxiolytic-like action of DuP753, a non-peptide angiotensin II receptor antagonist. Neuroreport 1: 20–21.
Berridge CW, Waterhouse BD (2003). The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev 42: 33–84.
Biggio G, Concas A, Corda MG, Giorgi O, Sanna E, Serra M (1990). GABAergic and dopaminergic transmission in the rat cerebral cortex: effects of stress, anxiolytic and anxiogenic drugs. Pharmacol Ther 48: 121–142.
Bittencourt JC, Sawchenko PE (2000). Do centrally administered neuropeptides access cognate receptors? An analysis in the central corticotropin-releasing factor system. J Neurosci 20: 1142–1156.
Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM (2003). Angiotensin II AT1 receptor blockade prevents gastric ulcers during cold-restraint stress. Am J Physiol 285: G414–G423.
Bremner JD, Innis RB, Southwick SM, Staib L, Zoghbi S, Charney DS (2000). Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related post traumatic stress disorder. Am J Psychiatry 157: 1120–1126.
Brunson KL, Grigoriadis DE, Lorang MT, Baram TZ (2002). Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain. Exp Neurol 176: 75–86.
Butler PD, Weiss JM, Stout JC, Nemeroff CB (1990). Corticotropin releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci 10: 176–183.
Carrasco GA, Van de Kar LD (2003). Neuroendocrine pharmacology of stress. Eur J Pharmacol 463: 235–272.
Castrén E, Saavedra JM (1988). Repeated stress increases the density of angiotensin II binding sites in the rat paraventricular nucleus and subfornical organ. Endocrinology 122: 370–372.
Chalmers DT, Lovenberg TW, De Souza EB (1995). Localization of novel corticotropin-releasing factor receptors (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 15: 6340–6350.
Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C et al (1986). Alterations in corticotropin-releasing factor like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci 6: 2908–2914.
Cullinan WE (2000). GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol 419: 344–351.
De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000). International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472.
Dunn AJ, Berridge CW (1990). Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev 15: 71–100.
Fernández-López A, Chinchetru MA, Fernández PC (1997). The autoradiographic perspective of central benzodiazepine receptors: a short review. Gen Pharmacol 29: 173–180.
Grima B, Lamouroux A, Blanot F, Biguet NF, Mallet J (1985). Complete coding sequence or rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci USA 82: 617–621.
Guo DF, Uno S, Ishihata A, Nakamura N, Inagami T (1995). Identification of a cis-acting glucocorticoid responsive element in the rat angiotensin II type 1A promoter. Circ Res 77: 249–257.
Herman JP, Figueiredo H, Mueller NK, Ultich-Lai Y, Ostrander MM, Choi DC et al (2003). Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24: 151–180.
Iredale PA, Terwilliger R, Widnell KL, Nestler EJ, Duman RS (1996). Differential regulation of corticotrophin-releasing factor 1 receptor expression by stress and agonist treatments in brain and cultured cells. Mol Pharmacol 50: 1103–1110.
Israel A, Strömberg C, Tsutsumi K, Garrido MDR, Torres M, Saavedra JM (1995). Angiotensin II receptor subtypes and phosphoinositide hydrolysis in rat adrenal medulla. Brain Res Bull 38: 441–446.
Jezova D, Ochedalski T, Kiss A, Aguilera G (1998). Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. J Neuroendocrinol 10: 67–72.
Jezova M, Armando I, Bregonzio C, Yu ZX, Qian S, Ferrans VJ et al (2003). Angiotensin II AT1 and AT2 receptors contribute to maintain basal adrenomedullary norepinephrine synthesis and tyrosine hydroxylase. Endocrinology 144: 2092–2101.
Jöhren O, Inagami T, Saavedra JM (1995). AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. Neuroreport 6: 2549–2551.
Kaiser FC, Palmer GC, Wallace AV, Carr RD, Fraser-Rae L, Hallam C (1992). Antianxiety properties of the angiotensin II antagonist, DUP 753, in the rat using the elevated plus-maze. Neuroreport 3: 922–924.
Keck ME, Holsboer F (2001). Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides 22: 835–844.
Koob GF (1999). Corticotropin releasing factor, norepinephrine and stress. Biol Psychiatry 46: 1167–1180.
Korte MS, De Boer SF (2003). A robust animal model of state anxiety: fear potentiated behavior in the elevated plus-maze. Eur J Pharmacol 463: 163–175.
Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C (1998). Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience 82: 827–841.
Leong DS, Terrón JA, Falcón-Neri A, Armando I, Ito T, Jöhren O et al (2002). Restraint stress modulates brain, pituitary and adrenal expression of angiotensin II AT1A, AT1B and AT2 receptors. Neuroendocrinology 75: 227–240.
Lippa AS, Klepner CA, Yunger L, Sano MC, Smith WV, Beer B (1978). Relationship between benzodiazepine receptors and experimental anxiety in rats. Pharmacol Biochem Behav 9: 853–856.
Lister RG (1987). The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 92: 180–185.
McCarthy JR, Heindrichs SC, Grigoriadis DE (1999). Recent advances with the CRF1 receptor: design of small molecule inhibitors, receptor subtypes and clinical indications. Curr Pharm Des 5: 289–315.
Medina JH, Novas ML, Wolfman CN, Levi de Stein M, De Robertis E (1983). Benzodiazepine receptors in rat cerebral cortex and hippocampus undergo rapid and reversible changes after acute stress. Neuroscience 9: 331–335.
Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, Koob GF (1994). Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther 269: 564–572.
Millan MJ, Brocco M, Gobert A, Dorey G, Casara P, Dekeyne A (2001). Anxiolytic properties of the selective, non-peptidergic CRF1 antagonists, CP154526 and DMP695: a comparison to other classes of anxiolytic agent. Neuropsychopharmacology 25: 585–600.
Miller JA, Zahniser NR (1987). The use of 14C-labeled tissue paste standards for the calibration of 125I-labeled ligands in quantitative autoradiography. Neurosci Lett 81: 345–350.
Montgomery KC (1955). The relation between fear induced by novel stimulation and exploratory behavior. J Comp Psychol 48: 254–260.
Nazarali AJ, Gutkind JS, Saavedra JM (1989). Calibration of [125I]-polymer standards with [125I] brain paste standards for use in quantitative receptors autoradiography. J Neurosci Methods 30: 247–253.
Negro M, Fernández-López A, Calvo P (1995). Autoradiographical study of types 1 and 2 of benzodiazepine receptors in rat brain alter chronic ethanol treatment and its withdrawal. Neuropharmacology 34: 1177–1182.
Nishimura Y, Ito T, Hoe K-L, Saavedra JM (2000). Chronic peripheral administration of the angiotensin II AT1 receptor antagonist candesartan blocks brain AT1 receptors. Brain Res 871: 29–38.
Nutt DJ, Malizia AL (2001). New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry 179: 390–394.
Okuyama S, Sakagawa T, Chaki S, Imagawa Y, Ichiki T, Inagami T (1999). Anxiety-like behavior in mice lacking the angiotensin II type-2 receptor. Brain Res 821: 150–159.
Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ (2001). Efferent neural projections of angiotensin receptor (AT1) expressing neurons in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13: 139–146.
Orchinik M, Carroll SS, Li HY, McEwen BS, Weiland NG (2001). Heterogeneity of hippocampal GABA(A) receptors: regulation by corticosterone. J Neurosci 21: 330–339.
Paxinos G, Watson C (1986). The Rat Brain in Stereotaxic Coordinates. Academic Press: New York.
Peng JF, Phillips MI (2001). Opposite regulation of brain angiotensin type1 and type 2 receptors in cold-induced hypertension. Regul Peptides 97: 91–102.
Phillips MI (1997). Functions of angiotensin in the central nervous system. Annu Rev Physiol 3: 103–126.
Primus RJ, Yevich E, Baltazar C, Gallager DW (1997). Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology 17: 308–316.
Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA (2004). Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci 21: 6545–6552.
Rodgers RJ, Johnson NJ (1998). Behaviorally selective effects of neuroactive steroids on plus-maze anxiety in mice. Pharmacol Biochem Behav 59: 221–232.
Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo Pich E, Rivier J, Koob GF et al (1996). Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38, Calpha MeLeu37] CRF attenuates the actions of the highly potent cannabinoid receptor agonist HU-210 on defensive withdrawal behavior in rats. J Pharmacol Exp Ther 276: 56–64.
Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R (1998). Characterization of [125I] Sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther 286: 459–468.
Saavedra JM (1992). Brain and pituitary angiotensin. Endocr Rev 18: 21–53.
Saavedra JM (1999). Emerging features of brain angiotensin receptors. Regul Peptides 85: 31–45.
Sánchez MM, Young LJ, Plotsky PM, Insel TR (1999). Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol 408: 365–377.
Schulz DM, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M et al (1996). CP-154526: a potent and selective antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci USA 93: 10477–10482.
Seltzer A, Bregonzio C, Armando I, Baiardi G, Saavedra JM (2004). Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res 1028: 9–18.
Serra M, Concas A, Mostallino MC, Chessa MF, Stomati M, Petraglia F et al (1999). Antagonism by pivagabine of stress-induced changes in GABAA receptor function and corticotropin-releasing factor concentrations in rat brain. Psychoneuroendocrinology 24: 269–284.
Shimizu N, Nakane H, Hori T, Hayashi Y (1994). CRF receptor antagonist attenuates stress-induced noradrenaline release in the medial prefrontal cortex of rats. Brain Res 654: 145–148.
Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH et al (1998). Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20: 1093–1102.
Sumitomo T, Suda T, Nakano Y, Tozawa F, Yamada M, Demura H (1991). Angiotensin II increases the corticotropin-releasing factor messenger ribonucleic acid levels in the rat hypothalamus. Endocrinology 128: 2248–2252.
Szafarczyk A, Feuvrier E, Siaud P, Rondouin G, Lacoste M, Gaillet S et al (1995). Removal of adrenal steroids from the medium reverses the stimulating effects of catecholamines on corticotropin-releasing hormone neurons in organotypic cultures. Neuroendocrinology 61: 517–524.
Takamatsu Y, Yamamoto H, Ogunremi OO, Matsuzaki I, Moroji T (1991). The effects of corticotropin-releasing hormone on peptidergic neurons in the rat forebrain. Neuropeptides 20: 255–265.
Tsutsumi K, Saavedra JM (1991a). Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol 26: 209–216.
Tsutsumi K, Saavedra JM (1991b). Angiotensin II receptor subtypes in median eminence and basal forebrain areas involved in the regulation of pituitary function. Endocrinology 129: 3001–3008.
Valdez GR, Sabino V, Koob GF (2004). Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res 28: 865–972.
Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C et al (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212.
Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP (1996). In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology 137: 5747–5750.
Weizman R, Weizman A, Kook KA, Vocci F, Deutsch SI, Paul SM (1989). Repeated swim stress alters brain benzodiazepine receptors measured in vivo. J Pharmacol Exp Ther 249: 701–707.
Whitnall MH (1993). Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol 40: 573–629.
Wisden W, Morris BJ (1994). In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ (eds). In Situ Hybridization Protocols for the Brain. Academic Press: San Diego. pp 9–34.
Xang G, Xi ZX, Wan Y, Wang H, Bi G (1993). Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signals 2: 166–172.
Yang G, Wan Y, Zhu Y (1996). Angiotensin II—an important stress hormone. Biol Signals 5: 1–8.
Zavala F (1997). Benzodiazepines, anxiety and immunity. Pharmacol Ther 75: 199–216.
Acknowledgements
We thank ASTRA Sweden for the supply of candesartan. This research was supported by the Intramural Research Program of the National Institute of Mental Health, NIH, DHHS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saavedra, J., Armando, I., Bregonzio, C. et al. A Centrally Acting, Anxiolytic Angiotensin II AT1 Receptor Antagonist Prevents the Isolation Stress-Induced Decrease in Cortical CRF1 Receptor and Benzodiazepine Binding. Neuropsychopharmacol 31, 1123–1134 (2006). https://doi.org/10.1038/sj.npp.1300921
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300921
Keywords
This article is cited by
-
Dynamic influences on the neural encoding of social valence
Nature Reviews Neuroscience (2022)
-
The AT-1 Angiotensin Receptor is Involved in the Autonomic and Neuroendocrine Responses to Acute Restraint Stress in Male Rats
Cellular and Molecular Neurobiology (2022)
-
The Angiotensin Type 1 Receptor Antagonist Losartan Prevents Ovariectomy-Induced Cognitive Dysfunction and Anxiety-Like Behavior in Long Evans Rats
Cellular and Molecular Neurobiology (2020)
-
Central blockade of the AT1 receptor attenuates pressor effects via reduction of glutamate release and downregulation of NMDA/AMPA receptors in the rostral ventrolateral medulla of rats with stress-induced hypertension
Hypertension Research (2019)
-
AT1 and AT2 Receptors in the Prelimbic Cortex Modulate the Cardiovascular Response Evoked by Acute Exposure to Restraint Stress in Rats
Cellular and Molecular Neurobiology (2018)