Abstract
The discriminative stimulus properties of ethanol are mediated in part by positive modulation of GABAA receptors. Recent evidence indicates that metabotropic glutamate receptor subtype 5 (mGluR5) activity can influence GABAA receptor function. Therefore, the purpose of this work was to examine the potential involvement of mGluR5 in the discriminative stimulus effects of ethanol. In rats trained to discriminate ethanol (1 g/kg, intragastric gavage (i.g.)) from water, 2-methyl-6-(phenylethyl)-pyridine (MPEP) (1–50 mg/kg, i.p.) a selective noncompetitive antagonist of the mGlu5 receptor did not produce ethanol-like stimulus properties. However, pretreatment with MPEP (30 mg/kg) reduced the stimulus properties of ethanol as indicated by significant reductions in ethanol-appropriate responding, specifically at 0.5 and 1 g/kg ethanol, and a failure of ethanol test doses (1 and 2 g/kg) to fully substitute for the ethanol training dose. To test whether mGluR5 antagonism altered the GABAA receptor component of the ethanol stimulus, the ability of MPEP to modulate pentobarbital and diazepam substitution for ethanol was assessed. Pentobarbital substitution (1–10 mg/kg, i.p.) for ethanol was not altered by MPEP pretreatment. However, MPEP pretreatment inhibited the ethanol-like stimulus properties of diazepam (5 mg/kg, i.p.). To examine a potential anatomical basis for these pharmacological findings, expression patterns of mGluR5- and benzodiazepine-sensitive GABAA α1-containing receptors were examined by dual-label fluorescent immunohistochemistry with visualization by confocal microscopy. Results indicated that mGluR5- and GABAA α1-containing receptors were both coexpressed in limbic brain regions and colocalized on the same cells in specific brain regions including the amygdala, hippocampus, globus pallidus, and ventral pallidum. Together, these findings suggest an interaction between mGluR5- and benzodiazepine-sensitive GABAA receptors in mediating ethanol discrimination.
Similar content being viewed by others
INTRODUCTION
The amino-acid glutamate is the primary excitatory transmitter in the mammalian CNS. Fast excitatory actions of glutamate are mediated by ionotropic glutamate receptor (iGluR) N-methyl-D-aspartate (NMDA), α-amino-3-hydroxi-5-methyl-ioxyzole-4-propionic acid, and kainate receptors. Metabotropic glutamate receptors (mGluRs) mediate slower glutamate responses through G-protein coupling to various intracellular signaling cascades that can modulate, or fine-tune, iGluR function (Benquet et al, 2002). Eight mGluR subtypes (mGluR1–8) have been cloned. These receptors have been classified into three groups based on amino-acid sequence similarity, agonist pharmacology, and the signal transduction pathways to which they couple. Group I mGluRs (mGluR1 and 5) upregulate Ca2+ cascades, whereas Group II (mGluR2 and 3) and Group III (mGluR4 and 6–8) receptors are both negatively coupled to the cAMP cascade but are distinguished by their differing agonist–antagonist profiles and sequence homologies (Gereau and Conn, 1995).
The availability of selective pharmacological agents has quickly begun to reveal basic functional roles of mGluR5. MGluR5 activation with a selective agonist (CHPG) elevates glutamate release (Pintor et al, 2000) and augments NMDA-induced membrane depolarization in striatal slices (Pisani et al, 2001). Other evidence indicates that mGluR5 contributes to striatal synaptic plasticity since the mGluR5 selective antagonist, 2-methyl-6-(phenylethyl)-pyridine (MPEP), decreased long-term depression at corticostriatal synapses (Sung et al, 2001). Other studies have found beneficial effects of mGluR5 antagonists in animal models of anxiety, pain, and neurodegeneration (Bordi and Ugolini, 1999; Spooren et al, 2001). Moreover, genetic and pharmacological data have started to reveal a role for mGluR5 in addiction. For instance, morphine (10 mg/kg) conditioned reward is inhibited by MPEP (30 mg/kg) (Popik and Wrobel, 2002) but lower doses of MPEP do not alter morphine conditioned place preference (McGeehan and Olive, 2003). Mice lacking the mGluR5 gene do not self-administer cocaine and show no cocaine-induced increase in locomotor activity (Chiamulera et al, 2001), indicating a significant role of mGluR5 in the behavioral effects of psychomotor stimulants.
Many of the physiological, biochemical, and behavioral effects of ethanol are known to involve iGluR function (Dodd et al, 2000; Mihic, 1999; Aschner et al, 2001; Woodward, 1999; Tabakoff and Hoffman, 1993; Littleton et al, 2001; Weight et al, 1993; Costa et al, 2000). In addition to its effects on iGluRs, however, ethanol also modulates general metabotropic receptor activity as evidenced by its ability to reduce basal and stimulated phosphoinositide hydrolysis (Gonzales et al, 1986). Low concentrations of ethanol selectively alter neuronal firing rates (Netzeband and Gruol, 1995) and Ca2+ levels (Gruol et al, 1997) mediated by mGluRs in vitro. Chronic exposure to ethanol reduces mGluR1 mRNA levels in cerebellar Purkinje neurons of mice (Simonyi et al, 1996) and early withdrawal from ethanol leads to alterations in mGluR-evoked Ca2+ signaling in cerebellar neurons (Netzeband et al, 2002). Moreover, pharmacologically relevant concentrations of ethanol inhibit glutamate-induced Ca2+-dependent Cl− currents in Xenopus oocytes expressing mGluR5, but have no effect on currents in oocytes expressing mGluR1 (Minami et al, 1998), suggesting that ethanol may selectively alter mGluR5 function. In addition, the mGluR5 antagonist MPEP reduces the reinforcing properties of ethanol (Sharko et al, 2002), and reduces alcohol relapse to alcohol self-administration in two animal models (Bäckström et al, 2004).
In addition to their reinforcing properties, drugs of abuse produce distinctive subjective (ie discriminative stimulus) effects that are an important determinant of abuse liability (Stolerman, 1992). Using differential reinforcement methods, drug discrimination experiments involve training animals (eg pigeons, mice, rats, gerbils, monkeys, humans) to emit one response (ie a lever press) after being injected with one dose of drug (ie called the training drug or training dose). Thus, reinforcement (food or sucrose) is contingent on selecting the ‘correct’ or ‘drug-appropriate’ lever. An alternative response (ie pressing a different lever) results in reinforcement after vehicle injection. Once this discriminated performance is established, other doses of the training drug, or other drugs, are administered to determine if they engender responding on the drug-appropriate lever. The extent to which a novel dose, or novel drug, produces responding on the lever associated with the training drug is called drug ‘substitution’ or ‘generalization.’ The interpretation of these results is that a drug that produces high levels of responding on the lever associated with the training drug (ie ethanol) has similar subjective properties and acts by similar neurobiological mechanism(s) (Grant and Colombo, 1993; Kostowski and Bienkowski, 1999). Novel drugs can also be tested for pharmacological potentiation or antagonism of the discriminative stimulus effects of the training drug by conducting pretreatment experiments (Besheer et al, 2003).
The discriminative stimulus effects of ethanol are mediated at least by GABAA receptors. For example, positive modulators of GABAA receptors, such as barbiturates, benzodiazepines, and neurosteroids, produce ethanol-like stimulus effects (Ator et al, 1993; Barry, 1991; Evans and Balster, 1991; Grant et al, 1996, 1997; Jarbe and McMillan, 1983; Shelton and Grant, 2002) in multiple species. MGluR5 activity has the potential to influence ethanol discrimination based on evidence that mGluR5 can influence GABA neurotransmission (de Novellis et al, 2003; Hoffpauir and Gleason, 2002; Diaz-Cabiale et al, 2002). For example, the mGluR5 agonist CHPG has been shown to increase GABA levels in vivo and antagonism of mGlu5 receptors blocks the CHPG-induced increases in GABA (de Novellis et al, 2003). In cultured amacrine cells, GABA-gated currents are enhanced by CHPG administration, an enhancement that is likely due to postsynaptic GABAA receptors (Hoffpauir and Gleason, 2002). MGlu5 receptors are abundant in limbic brain regions such as the nucleus accumbens, cortex, and hippocampus (Bordi and Ugolini, 1999; Spooren et al, 2001; Romano et al, 1995), where GABAA receptors are known to modulate ethanol discrimination. For example, site-specific infusion of GABAA-positive modulators such as muscimol, pentobarbital, or allopregnanolone in the nucleus accumbens or amygdala substitute fully for systemic ethanol (Besheer et al, 2003; Hodge and Cox, 1998; Hodge et al, 2001). It is plausible, therefore, that mGlu5 receptors might regulate ethanol discrimination via modulation of GABAA receptor function.
Thus, the purpose of this study was to determine the potential involvement of mGluR5 in the modulation of the discriminative stimulus effects of ethanol. In rats trained to discriminate (1 g/kg) ethanol from water, MPEP a noncompetitive antagonist of the mGlu5 receptor (Gasparini et al, 1999) was assessed for its ability to substitute for ethanol and to modulate ethanol discrimination. Further, the ability of MPEP to modulate pentobarbital and diazepam substitution for ethanol was assessed. Finally, to examine the potential anatomical basis for an interaction between mGlu5 and GABAA receptors, expression patterns of these receptors were examined using dual-label florescent immunohistochemistry with confocal visualization.
METHOD
Animals
In all, 12 male Long Evans rats (Harlan Sprague–Dawley, Indianapolis, IN) weighing 150–200 g upon arrival to the colony were individually housed in Plexiglas cages. Rats were handled and weighed daily for 2 weeks before lever press training began. Once rats reached a body weight of approximately 300 g, rats were fed 16 g of food daily for the remainder of the 12-month study (weights maintained at approximately 325 g). Water was available continuously in the home cage. The colony room was maintained on a 12-h light/dark cycle and experiments were conducted during the light portion of the cycle. Animals were under continuous care and monitoring by veterinary staff from the Division of Laboratory Animal Medicine (DLAM) at UNC-Chapel Hill, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). DLAM staff considered all of the rats to be healthy throughout the course of the experiment, indicating that the procedures produced no adverse affects on animal health or well-being. All procedures were also carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
Apparatus
Operant chambers (Med Associates, Georgia, VT) measuring 31 × 32 × 24 cm3 were used for discrimination sessions. The chambers were located within sound-attenuating cubicles and equipped with an exhaust fan that provided ventilation and masked external sounds. Two response levers were located on the right wall of each chamber. Responses on the levers activated a liquid dispenser centered between the levers that presented fluid in a 0.1 ml dipper cup for 4 s during each operation. A stimulus light was located above each response lever and was activated each time a reinforcer was delivered. The chambers were illuminated by an 8-W light located on the left wall 28 cm above the floor. The chambers were interfaced (Med Associates) to a computer that was programmed to control sessions and record data.
Procedure
Lever press training
Rats were trained to lever press on a fixed ratio 1 (FR1) schedule of reinforcement in overnight sessions. That is, a single lever press on either of the two levers resulted in presentation of 0.1 ml of a liquid sucrose solution (10% (w/v)). After three overnight sessions, 1 h lever press training began. During these sessions, rats were placed in the chamber for 10 min before the house light was illuminated and either the right or the left lever was extended into the chamber and signaled the beginning of the session. The presentation of the right and left lever was alternated each day and the schedule of reinforcement was gradually increased to FR10. That is, once 10 lever presses occurred, the sucrose was presented for 4 s. Rats received equal experience with both levers at each reinforcement schedule. Discrimination training began once responding on the FR10 schedule was stable (<10% daily variation in the total number of responses).
Discrimination training
Training sessions were conducted 5 days per week (M–F) during which ethanol (1 g/kg) or water was administered by intragastric gavage (i.g.) prior to the start of the 15-min sessions. Immediately following the ethanol or water administration, the rats were placed in the chambers. Similar to lever press training sessions, after 10 min the house light was illuminated and both levers were introduced into the chamber signaling the beginning of the session. Following ethanol administration, completion of 10 responses on the ethanol-appropriate lever resulted in the presentation of the sucrose solution. Similarly, following water administration, completion of 10 responses on the water-appropriate lever resulted in sucrose delivery. During both the ethanol and water sessions, responses on the inappropriate lever were recorded but produced no programmed consequences. The lever associated with ethanol or water administration was randomly assigned and counterbalanced across animals. Water and ethanol training days varied on a double alternation schedule (W, W, E, E, etc.). The training sessions continued until the percentage of ethanol- and water-appropriate lever press responses emitted prior to the first reinforcer, and during the entire session was >80% for 10 consecutive days. Once these criteria were met, testing began.
Testing procedures
Test sessions were identical to the training sessions, except that they were 2 min in duration, and completion of an FR10 on either lever resulted in sucrose delivery. Reinforcement was delivered during test sessions to assess the possible effects of test treatments on overall response rates. These test sessions were interspersed with training sessions only if performance during the previous five training sessions met the accuracy criteria. If the criteria were not met, training continued until response accuracy was >80% for 5 consecutive days, consequently, not all rats were used in each test condition.
Substitution Testing
Ethanol substitution
In four different test sessions, various ethanol doses (0.1, 0.5, 1, and 2 g/kg, i.g.) were administered to determine an ethanol substitution curve. Rats (n=12) received each ethanol dose in a random order.
MPEP substitution
To test whether MPEP would substitute for ethanol, rats (n=6) were administered MPEP (0, 1, 10, 30, and 50 mg/kg, i.p.) 20 min before receiving a water gavage and were then placed in the chambers. Each MPEP dose was administered once in random order.
Ethanol substitution with MPEP pretreatment
In order to evaluate the effects of MPEP pretreatment on the stimulus effects of multiple ethanol doses, rats (n=8) were administered MPEP (30 mg/kg, i.p.) or saline 20 min before ethanol (0.1, 0.5, 1, and 2 g/kg, i.g.). Rats were tested once on each pretreatment combination and the combinations were tested randomly.
GABAA-positive modulator substitution with MPEP pretreatment
Saline or MPEP (30 mg/kg, i.p.) was administered 20 min before pentobarbital injection (1, 3, and 10 mg/kg, i.p.). Rats (n=10) were tested once on each pretreatment combination and the combinations were tested randomly. After pentobarbital testing, saline or MPEP (30 mg/kg, i.p.) was administered 20 min before diazepam injection (1, 3, and 5 mg/kg, i.p.). Rats (n=11) were tested once on each pretreatment combination and the combinations were tested randomly. Gavage was withheld during these tests.
Drugs
Ethanol (95% (w/v)) was diluted in distilled water to a concentration of 20% (v/v) and was administered i.g. in various volumes to obtain doses of 0.1, 0.5, 1, and 2 g/kg. A corresponding volume of water to the 1 g/kg ethanol training dose was used. Pentobarbital (Sigma-Aldrich, St Louis, MO) was dissolved in saline, and injected i.p. at a volume of 1 ml/kg. MPEP (Sigma) was also dissolved in saline by gently heating the solution and injected i.p. at a volume of 1 ml/kg, except for the 50 mg/kg dose, which was injected at a volume of 2 ml/kg. Diazepam (Sigma) in a suspension of 1% carboxymethylcellulose was injected i.p. at a volume of 2 ml/kg.
Data Analysis
Response accuracy was expressed as the percentage of ethanol-appropriate lever presses upon delivery of the first reinforcer. Response rate (responses/min) was analyzed for the entire session and provided an index of locomotor ability. Complete substitution for the ethanol stimulus was defined as >80% choice of the ethanol lever upon completion of the first FR10 during test sessions. If an animal did not complete an FR10 during these test sessions, then that animal was not included in the response accuracy analysis, but was included in the response rate analysis. The left-most panels of each figure show the percentage of ethanol-appropriate lever responding on completion of the first FR10 and response rate during the ethanol and water session that preceded testing of that drug combination. One or two-way repeated measures analysis of variance (ANOVA) were used to analyze response accuracy and response rate data. Planned comparisons were made within each pretreatment condition (eg each diazepam dose compared to lowest diazepam dose in the saline pretreatment condition), and between the pretreatment conditions at each test drug dose (eg responses after saline and MPEP compared at each diazepam dose). ED50 values for the dose effects were determined by log-dose probit analysis when possible, and paired t-tests were used to compare the ED50's for the ethanol, pentobarbital, and diazepam tests.
Immunohistochemistry
At the conclusion of the ethanol discrimination study, the animals (n=10) were deeply anesthetized with pentobarbital (100 mg/kg, i.p.) and perfused transcardially with 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. The brains were removed from the skull and placed in the same fixative solution for at least 24 h before being washed with PBS and sliced on a vibratome into 40 μm sections. Primary antibodies used in the study were a rabbit polyclonal antibody raised against mGluR5 (Neuromics, Northfield, MN; dilution 1:300) and a guinea-pig polyclonal antibody raised against GABAA α1-subunit (courtesy of Dr Jean-Marc Fritschy; dilution 1:300). The fluorescent-coupled secondary antibodies were Alexa Fluor® 488 goat anti-rabbit IgG (1:200 dilution) for mGluR5 and Alexa Fluor® 594 goat anti-guinea pig IgG (1:200 dilution) for GABAA α1 (Molecular Probes, Eugene, OR, USA). Visualization was performed using a Zeiss 410 laser-scanning confocal microscope (Carl Zeiss, Oberkochen, Germany). Images were saved in tagged image format and exported to Adobe Photoshop CS (Adobe Systems, Mountain View, CA) for analysis.
RESULTS
Ethanol Substitution
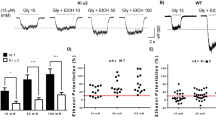
The left-most panels of Figure 1a and b show the percentage of ethanol-appropriate lever responding on completion of the first FR10 and response rate during the ethanol and water session that preceded testing of the ethanol dose response. Responding on the ethanol-appropriate lever during the ethanol session was approximately 96% and less than 2% on the water session. The percentage of ethanol-appropriate responses increased significantly as a function of ethanol test dose (F(3,47)=52.31, p<0.001), indicating that the procedures established reliable stimulus control. Both the 1 and 2 g/kg test doses of ethanol substituted fully for the 1 g/kg training dose (Figure 1a), with an ED50 value of 0.56 g/kg (±0.06 SEM). The training dose (1 g/kg) did not affect response rate (Figure 1b). However, a significant reduction in lever press responding was observed (F(3,47)=5.85, p=0.003), with the highest dose (2 g/kg) suppressing responding relative to the lowest dose tested.
(a) Mean (±SEM) percentage of ethanol-appropriate responding upon completion of the first FR10 at each ethanol dose tested (n=12). (b) Mean (±SEM) test session response rate at each ethanol dose tested. Data points to the left of the x-axis break represent performance on the water (W) and ethanol (E) training session prior to testing. Data points to the right of the x-axis break represent test session performance following IG ethanol administration. The horizontal dashed line (>80%) represents full substitution for the discriminative stimulus effects of ethanol (1 g/kg, i.g.). *Significant difference from 0.1 g/kg ethanol (Tukey's, p<0.05).
MPEP Substitution
As shown in Figure 2a, MPEP did not substitute for the stimulus effects of 1 g/kg ethanol (F(4,20)=1.58, p=0.22). All MPEP doses tested failed to substitute for ethanol. The highest MPEP dose tested (50 mg/kg) reduced response rate relative to saline (F(4,20)=2.91, p=0.047).
(a) Mean (±SEM) percentage of ethanol-appropriate responding upon completion of the first FR10 at each MPEP dose tested (n=6). (b) Mean (±SEM) test session response rate at each MPEP dose tested. Data points to the left of the x-axis break represent performance on the water (W) and ethanol (E) training session prior to testing. Data points to the right of the x-axis break represent test session performance following i.p. MPEP administration. The horizontal dashed line (>80%) represents full substitution for the discriminative stimulus effects of ethanol (1 g/kg, i.g.). *Significant difference from saline (Tukey's, p<0.05).
Ethanol Substitution with MPEP Pretreatment
Figure 3a shows the ethanol dose–response curve following saline and MPEP pretreatment. MPEP pretreatment at the 1 and 2 g/kg ethanol doses includes seven animals due to the death of one animal before testing was completed. Similar to the previous ethanol dose–response curve presented in Figure 1a, dose-dependent substitution for the ethanol training dose (1 g/kg) was observed. This dose-dependent substitution was inhibited by MPEP pretreatment. The two-way repeated measures ANOVA revealed a main effect of ethanol dose (F(3,21)=20.57, p<0.001). After saline pretreatment, ethanol-appropriate responding was greater at every ethanol dose (0.5, 1, and 2 g/kg) relative to the 0.1 g/kg ethanol dose, p's<0.03. MPEP pretreatment significantly altered this pattern of responding, as only the highest ethanol dose (2 g/kg) produced significantly greater ethanol-appropriate responding than the 0.1 g/kg ethanol dose, p=0.002. There was a significant main effect of pretreatment injection (F(1,7)=15.37, p=0.005). MPEP pretreatment significantly reduced ethanol-appropriate responding at the 0.5 and 1 g/kg ethanol doses, p's<0.001. The ethanol dose by pretreatment injection interaction was also significant, (F(3,19)=4.62, p=0.01). The mean ED50±SEM for ethanol substitution was 0.39±0.08 g/kg following saline pretreatment and 1.33±0.19 g/kg following MPEP pretreatment. This 3.4-fold rightward shift in the ethanol dose–response curve was significant, (t(3)=5.4 p=0.01).
(a) Mean (±SEM) percentage of ethanol-appropriate responding upon completion of the first FR10 after saline or MPEP pretreatment at each ethanol dose (n=8). (b) Mean (±SEM) test session response rate after saline or MPEP pretreatment at each ethanol dose tested. Data points to the left of the x-axis break represent performance on the water (W) and ethanol (E) training session prior to testing. The horizontal dashed line (>80%) represents full substitution for the discriminative stimulus effects of ethanol (1 g/kg, i.g.). *Significantly different from the respective 0.1 g/kg ethanol dose within pretreatment condition. †Significant difference between saline and MPEP pretreatment (Tukey's, p<0.05).
As shown in Figure 3b, response rate was dose dependently reduced by ethanol (F(3,21)=6.38, p=0.003), with significant rate reductions observed at the highest ethanol dose (2 g/kg) relative to the lowest dose (0.1 g/kg) after saline and MPEP pretreatment, p's<0.02. The main effect of pretreatment injection was also significant (F(1,7)=7.58, p=0.03), but no significant interaction was observed. At the lowest ethanol dose tested (0.1 g/kg), response rate was significantly lower after MPEP pretreatment, p=0.02. MPEP did not induce a rate reduction at any other ethanol dose. Taken together, MPEP pretreatment reduced ethanol-appropriate responding, at doses that were not affected by response rate reductions (0.5 and 1 g/kg).
Pentobarbital Substitution for Ethanol with MPEP Pretreatment
Pentobarbital dose dependently substituted for ethanol as shown in Figure 4a. Two rats did not complete a first FR when MPEP was administered before the highest pentobarbital dose (10 mg/kg). Consequently, those animals are not included in the response accuracy analysis (Figure 4a), but are included in the response rate analyses (Figure 4b). The two-way repeated measures ANOVA revealed a main effect of pentobarbital dose (F(2,18)=23.13, p<0.001), with the highest pentobarbital dose (10 mg/kg) producing significantly greater responding on the ethanol-appropriate lever than the lowest dose (1 mg/kg) after saline and MPEP pretreatment, p's<0.002. Pentobarbital substitution was not altered by MPEP pretreatment, as confirmed by the lack of a significant main effect of pretreatment injection (F<1). The pentobarbital dose by pretreatment injection interaction was also not significant (F<1). The mean±SEM ED50 for pentobarbital substitution was 5.26±0.81 mg/kg following saline pretreatment and 4.89±0.85 mg/kg following MPEP pretreatment.
(a) Mean (±SEM) percentage of ethanol-appropriate responding upon completion of the first FR10 after saline or MPEP pretreatment at each pentobarbital dose tested (n=10). (b) Mean (±SEM) test session response rate after saline or MPEP pretreatment at each pentobarbital dose tested. Data points to the left of the x-axis break represent performance on the water (W) and ethanol (E) training session prior to testing. The horizontal dashed line (>80%) represents full substitution for the discriminative stimulus effects of ethanol (1 g/kg, i.g.). *Significantly different from the respective 1 mg/kg pentobarbital dose within pretreatment condition. †Significant difference between saline and MPEP pretreatment (Tukey's, p<0.05).
Response rate was significantly altered by pentobarbital dose (F(2,18)=17.05, p<0.001), with a significant reduction observed at the 10 mg/kg dose after saline and MPEP pretreatment, p's<0.001 (Figure 4b). Response rate was also reduced by MPEP pretreatment (F(1,9)=15.46, p=0.003), and at each pentobarbital dose MPEP decreased responding relative to saline pretreatment, p's<0.03. The pentobarbital dose by pretreatment injection interaction was not significant (F<1).
Diazepam Substitution for Ethanol with MPEP Pretreatment
Diazepam dose dependently substituted for ethanol as shown in Figure 5a. One animal died before completion of the tests, and accordingly, the 1 mg/kg diazepam dose following MPEP pretreatment includes data from 10 animals. The two-way repeated measures ANOVA revealed a significant main effect of diazepam dose (F(2,20)=4.94, p=0.02). After saline pretreatment, significantly greater responding on the ethanol-appropriate lever at the highest diazepam dose (5 mg/kg) relative to the 1 mg/kg diazepam dose was evident, p=0.002. This effect was prevented by MPEP pretreatment. The main effect of pretreatment injection was not significant; however, the diazepam dose by pretreatment injection interaction was significant (F(2,19)=3.72, p=0.04). MPEP pretreatment reduced ethanol-appropriate responding at the 5 mg/kg diazepam dose relative to saline pretreatment, p=0.01. The mean±SEM ED50 for diazepam substitution was 2.23±0.21 mg/kg and was 2.03±0.26 mg/kg following MPEP pretreatment. Given that MPEP pretreatment reduced diazepam substitution, the ED50 following MPEP pretreatment was determined from the data of five animals.
(a) Mean (±SEM) percentage of ethanol-appropriate responding upon completion of the first FR10 after saline or MPEP pretreatment at each diazepam dose tested (n=11). (b) Mean (±SEM) test session response rate after saline or MPEP pretreatment at each diazepam dose tested. Data points to the left of the x-axis break represent performance on the water (W) and ethanol (E) training session prior to testing. The horizontal dashed line (>80%) represents full substitution for the discriminative stimulus effects of ethanol (1 g/kg, i.g.). *Significantly different from the respective 1 mg/kg diazepam dose within pretreatment condition. †Significant difference between saline and MPEP pretreatment (Tukey's, p<0.05).
Figure 5b illustrates response rate for the diazepam substitution tests. There was a significant main effect of diazepam dose (F(2,20)=4.69, p=0.02), with a response reduction observed after saline pretreatment at the highest diazepam dose (5 mg/kg) relative to the lowest diazepam dose (1 mg/kg), p=0.006. MPEP pretreatment also significantly reduced response rate (F(1,10)=11.14, p=0.007); a significant reduction was observed at the 1 and 3 mg/kg diazepam doses. The diazepam dose by pretreatment drug interaction was not significant. Thus, MPEP pretreatment significantly reduced ethanol-appropriate responses at the 5 mg/kg diazepam dose. A response rate reduction was observed at this dose after saline pretreatment; however, MPEP pretreatment did not enhance that response rate reduction.
Immunohistochemistry with Confocal Microscopy
In order to determine if there is an anatomical basis for the pharmacological interaction between BZ-sensitive GABAA receptors and mGlu5 receptors, dual-label fluorescent immunohistochemistry with confocal microscopy was used to examine both the individual and joint expression patterns of mGluR5 and GABAA receptors expressing the α1 molecular subunit, which constitutes the majority of BZ-sensitive GABAA receptors (Mohler et al, 1997). Previous work has shown that GABAA α1 receptors and mGlu5 receptors are both expressed in brain regions known to modulate ethanol discrimination such as the nucleus accumbens, amygdala, and hippocampus (Romano et al, 1995; Hodge and Cox, 1998; Pirker et al, 2000; Schwarzer et al, 2001; Besheer et al, 2003). In the shell and core (Figure 6a) of the nucleus accumbens, relatively few cell bodies showed GABAA α1 immunoreactivity, consistent with Härtig et al, (1995). Several GABAA α1-labeled processes were evident with some processes visibly extending into the ventral pallidum. Consistent with the existing literature, diffuse mGluR5 staining was observed throughout the striatum including the core (Figure 6b) and shell regions of the nucleus accumbens. In the basolateral amygdala, moderate mGluR5 and GABAA α1 immunoreactivity was found with labeled cell bodies and processes. In the CA1, CA2, CA3, and dentate gyrus of the hippocampus, intense mGluR5 staining of cell bodies and processes was evident (Figure 6e). GABAA α1 staining was also found on the cell bodies and processes in the same regions of the hippocampal formation (Figure 6d). The pallidal regions (ventral pallidum and globus pallidus) were also examined given that mGluR5 and GABAA α1 are expressed in those regions (Lu et al, 1999; Pirker et al, 2000; Schwarzer et al, 2001; Poisik et al, 2003) and that activation of mGluR5 in the nucleus accumbens has been shown to increase GABA levels in the ventral pallidum (Diaz-Cabiale et al, 2002), suggesting a potential interaction between the two receptor systems in this brain area. In the present work, intense staining of both GABAA α1 and mGluR5 was found on cell bodies and processes in the ventral pallidum (Figure 6g and h) and the globus pallidus (Figure 6j and k).
Confocal images from a representative rat showing the α1-subunit of the GABAA receptor and mGluR5 in the core of the nucleus accumbens (AcbC; a–c), the CA3 region of the hippocampus (CA3; d–f), the ventral pallidum (VP; g–i), and the globus pallidus (GP; j–l). GABAA α1 labeling appears in red, and mGluR5 labeling appears in green. Images a–i taken at × 63 magnification; images j–l taken at × 40 magnification.
The coexpression of mGluR5 and GABAA α1 on neurons within these brain regions has not been examined previously. As illustrated in Figure 6f, i and l, mGluR5 and GABAA α1 were coexpressed on cells in all of the limbic brain regions examined, except for the nucleus accumbens (Figure 6c). Interestingly, the coexpression pattern was relatively consistent across the other brain regions. That is, intense mGluR5 immunoreactivity appeared to be on the nuclear membrane, whereas intense GABAA α1 staining was localized on cell surface membrane receptors and processes.
DISCUSSION
The discriminative stimulus effects of ethanol are mediated in part by positive modulation of GABAA receptor activity (Ator et al, 1993; Grant and Lovinger, 1995; Hodge and Cox, 1998; Kostowski and Bienkowski, 1999; Hodge et al, 2001; Shelton and Grant, 2002). Evidence indicates that mGlu5 receptors can modulate GABAA receptor function (Awad et al, 2000; Hoffpauir and Gleason, 2002) and extracellular GABA levels. Moreover, ethanol inhibits glutamate-induced Ca2+-dependent Cl− currents in Xenopus oocytes expressing mGluR5 (Minami et al, 1998). Together, these findings suggest that the GABAA receptor component of ethanol's stimulus effects may be modulated by mGluR5 activity.
The purpose of this work was to examine the involvement of mGlu5 receptors in the discriminative stimulus properties of ethanol. Long Evans rats were trained by differential reinforcement to discriminate ethanol (1 g/kg, i.g.) from water vehicle using standard two-lever operant drug discrimination procedures (Hodge and Cox, 1998; Hodge et al, 2001; Besheer et al, 2003). The discrimination training procedures established reliable stimulus control as ethanol dose dependently substituted for the training dose of ethanol (1 g/kg). Administration of the selective mGluR5 antagonist MPEP (0–50 mg/kg) did not produce ethanol-like stimulus effects when administered alone, as indicated by low ethanol-appropriate responding. The highest dose of MPEP (50 mg/kg) significantly decreased response rate, which indicates that the compound was behaviorally active in the dose range tested and supports previous studies that found locomotor suppression by high doses of MPEP (Spooren et al, 2000). These data demonstrate that antagonism of mGluR5 function alone was not sufficient to produce discriminative stimulus properties that correspond to ethanol (1 g/kg).
A primary finding of the present study is that mGluR5 antagonism inhibited the discriminative stimulus properties of ethanol. Under control conditions, test doses of ethanol (1 and 2 g/kg) substituted fully (ie >80% ethanol-lever selection) for the discriminative stimulus properties of the ethanol (1 g/kg) training dose. Following MPEP (30 mg/kg) pretreatment, these test doses failed to fully substitute for the training dose. In addition, administration of MPEP prior to test doses of ethanol shifted the ethanol substitution curve more than three-fold to the right. This MPEP-induced shift in the ED50 for ethanol substitution was attributable to significant reductions in ethanol-appropriate responding following ethanol (0.5 and 1 g/kg) test doses. Reductions in response rate were seen only after combination of MPEP with the highest dose of ethanol (2 g/kg) tested, indicating that the reduction in the discriminative stimulus properties of ethanol was not associated with locomotor impairment. These results suggest that the discriminative stimulus effects of ethanol may be mediated in part by mGluR5 function.
In order to determine if the mGluR5 antagonist reduced ethanol discrimination by altering the GABAA receptor component of the ethanol stimulus, the effect of MPEP pretreatment on pentobarbital and diazepam substitution for ethanol (1 g/kg) was examined. Consistent with previous reports, pentobarbital (Ator et al, 1993; Grant et al, 1996; Hodge et al, 2001; Shelton and Grant, 2002) and diazepam (Jarbe and McMillan, 1983; Ator et al, 1993; Kostowski and Bienkowski, 1999; Green-Jordan and Grant, 2000; Shelton and Grant, 2002) dose dependently substituted for the stimulus effects of ethanol. MPEP pretreatment did not alter pentobarbital (1–10 mg/kg) substitution for ethanol. However, MPEP enhanced the response rate-reducing effects of pentobarbital, indicating that the mGluR5 antagonist was behaviorally active and able to modulate the motor effects of pentobarbital. Although a higher MPEP dose would most likely further enhance the locomotor depressant effects of pentobarbital, rendering effects on ethanol-appropriate responding difficult to interpret, it remains possible that a lower MPEP dose may have altered pentobarbital substitution. Conversely, the ethanol-like stimulus properties of diazepam (5 mg/kg) were significantly inhibited by 30 mg/kg MPEP pretreatment. The ED50 for ethanol substitution by diazepam was not altered by MPEP administration. This was due to the selective inhibition of ethanol-appropriate responding following only the highest dose of diazepam (5 mg/kg) tested. MPEP did not alter response rate when coadministered with diazepam, indicating that inhibition of the ethanol-like stimulus effects of diazepam was not associated with motor impairment. Both pentobarbital and diazepam are positive modulators of GABAA receptors, and consequently share a similar mechanism of action. That is, both compounds potentiate the action of GABA at the GABAA receptor (Study and Barker, 1981; Steinbach and Akk, 2001; Skerritt and Macdonald, 1984). However, pentobarbital can also directly gate the GABAA receptor in the absence of GABA (Serafini et al, 2000; Akk and Steinbach, 2000; Bormann, 1988). Another difference between the two compounds is that benzodiazepines, like diazepam, have specific binding sites on GABAA receptors (see later), while a specific binding site(s) for the barbiturates, like pentobarbital, has not yet been determined (Serafini et al, 2000). Further, barbiturates have pharmacological actions on other ligand-gated ion channel receptors such as neuronal nicotinic acetylcholine receptors and inhibitory glycine receptors (for a review see Krasowski and Harrison, 1999). Thus, the lack of an effect of MPEP pretreatment (30 mg/kg) on pentobarbital substitution for ethanol could be due to nonspecific effects of pentobarbital. These findings suggest that the mGluR5 antagonist MPEP inhibited the discriminative stimulus effects of ethanol specifically by reducing, or otherwise interfering with, the component of ethanol discrimination that is mediated by benzodiazepine-sensitive GABAA receptors.
Evidence of an interaction between GABAA and mGlu5 receptors has been reported (Hoffpauir and Gleason, 2002). In that study, the activity of GABAA receptors in retinal amacrine cells was enhanced by mGluR5 activation, as evidenced by an increase in GABA-gated currents after administration of CHPG, an mGluR5 agonist. The mGlu5 receptor has also been shown to influence GABA levels. For example, using microdialysis techniques, de Novellis et al, (2003) found that activation of mGluR5 by perfusion of CHPG through the dialysis probe significantly enhanced extracellular levels of GABA in the periaquaductal gray of rats. Similarly, intra-accumbens administration of CHPG increased GABA levels in the ventral pallidum (Diaz-Cabiale et al, 2002). In both studies, MPEP pretreatment blocked the agonist-induced increases in GABA levels. Clearly, mGlu5 receptors can influence GABA functioning, a major mediating factor in the discriminative stimulus effects of ethanol. The MPEP-induced reduction in the stimulus properties of ethanol, and the ethanol-like properties of diazepam, may have been a result of altered GABAergic functioning by MPEP pretreatment. Given that pentobarbital substitution for ethanol was not affected, BZ-sensitive GABAA receptors may have been differentially affected by MPEP-induced alterations. To our knowledge, interaction between BZ-sensitive GABAA and mGlu5 receptors has not been examined previously.
A majority of BZ-sensitive GABAA receptors with high affinity for diazepam include the α1β2γ2-subunit combination. The α2β3γ2- and α3β3γ2-subunit combinations also express high affinity for diazepam but are less abundant throughout the brain (McKernan and Whiting, 1996). The BZ-binding site is generally thought to exist on the α-subunit (Fuchs et al, 1988; Stephenson et al, 1990). The GABAA α1-subunit is distributed in brain regions known to modulate ethanol discrimination (eg nucleus accumbens, amygdala, hippocampus; Pirker et al, 2000), as is the mGlu5 receptor (Romano et al, 1995), and these distribution patterns were also found in the immunohistochemical studies of the present work. Further, we found that mGluR5 and GABAA α1 were coexpressed on cell bodies in all of the limbic brain regions examined, with the exception of the nucleus accumbens. The lack of coexpression in the nucleus accumbens may be due to the relatively few GABAA α1 immunoreactive cell bodies, and the diffuse nature of the mGluR5 staining in that brain region. Regardless, the consistent pattern of coexpression on cells suggests that mGluR5- and GABAA α1-containing receptors might interact at the brain regional level.
We also observed coexpression of mGlu5 and GABAA α1 receptors on the same cells in brain regions that modulate the discriminative stimulus effects of ethanol such as the amygdala and hippocampus (Hodge and Cox, 1998; Hodge et al, 2001; Besheer et al, 2003). Representative cells from the hippocampus (CA3) are shown in Figure 6 (panel f). In those cells showing coexpression, intense mGluR5 immunoreactivity appeared to be on the nuclear membrane, whereas GABAA α1 was localized on the cell surface, or plasma membrane. This pattern of mGluR5 nuclear membrane distribution is consistent with O'Malley et al (2003). In that study, using confocal microscopy mGluR5 was colocalized with lamin B2, a nuclear membrane marker. Accordingly, mGlu5 receptors expressed on nuclear membranes can produce sustained oscillatory Ca2+ responses (O'Malley et al, 2003), which indicates that these receptors can modulate intracellular signaling. This suggests that mGlu5 receptors might modulate GABAA receptor function on specific cells via alterations in Ca2+-linked second messenger systems such as PKC (eg Hodge et al, 1999), which is a possible basis for the pharmacological interaction between mGlu5 and GABAA receptors seen in the present work.
In addition to finding mGluR5 and GABAA α1 coexpression in brain regions known to modulate ethanol discrimination, the same cellular coexpression pattern was also observed in the ventral pallidum and the globus pallidus. Interestingly, GABAA α1 receptors in the ventral pallidum are known to mediate ethanol self-administration (June et al, 2003), but the role of pallidal regions in ethanol discrimination has not been examined. In the present study, both the ventral pallidum and globus pallidus showed intense immunoreactivity for the GABAA α1-subunit, which is consistent with previous findings that showed prominent expression of the α1-subunit along with β2- and γ2-subunits (Schwarzer et al, 2001), and suggests that these brain regions mediate pharmacological sensitivity to diazepam. Thus, the mGluR5 antagonist tested in the present study may have inhibited both ethanol discrimination, and diazepam substitution for ethanol, by modulating GABAA receptor function within pallidal regions.
Other evidence suggests that mGlu5 and GABAA receptors may interactively modulate neural circuits among brain regions. For example, a majority of projection neurons from the nucleus accumbens to the ventral pallidum express mGluR5 (Lu et al, 1999), suggesting that GABAergic projections from the accumbens can be modulated by mGluR5 activity. Indeed, infusion of the mGluR5 agonist CHPG into the nucleus accumbens increased GABA levels in the ventral pallidum (Diaz-Cabiale et al, 2002). The nucleus accumbens plays a major role in mediating the discriminative stimulus effects of ethanol. GABAA-positive modulators such as muscimol and allopregnanolone, microinjected into the nucleus accumbens produce ethanol-like stimulus properties (Hodge and Cox, 1998; Hodge et al, 2001; Besheer et al, 2003). Therefore, there exists the possibility that the discriminative stimulus properties of ethanol are mediated in part by GABAergic projection neurons from the nucleus accumbens to the pallidal regions. Moreover, antagonism of mGluR5 in this pathway may have inhibited ethanol discrimination and diazepam substitution for ethanol by altering GABA neurotransmission in the ventral pallidum. Clearly, the potential role of nucleus accumbens projections and the cellular interactions between mGluR5 and GABAA α1 in modulating the stimulus effects of ethanol will need to be further examined. However, given the behavioral and immunohistochemical findings of the present work and the existing literature, the foundation exists to predict an interaction between mGluR5 and GABAA α1 receptors within limbic circuits.
In conclusion, drugs of abuse produce distinctive subjective effects in humans that are often described as ‘high’ or ‘euphoria’. These subjective effects play a major role in the onset and maintenance of drug-taking behaviors (Stolerman, 1992). Thus, examination of the discriminative stimulus effects of drugs is essential for the effective development of therapeutics designed to aide in drug abuse-related disorders. Indeed, MPEP pretreatment has been found to reduce the acute reinforcing effects of ethanol (Sharko et al, 2002) and relapse to ethanol-seeking behavior (Bäckström et al, 2004), suggesting that targeting the mGlu5 receptor system may be a potential therapeutic strategy for the treatment of alcohol-related disorders. The findings from the present work suggest a specific behavioral mechanism by which MPEP may reduce ethanol reinforcement and relapse. That is, MPEP appears to reduce the subjective (ie discriminative stimulus) properties of ethanol, which may contribute to reductions in chronic drinking or excessive consumption during relapse. Moreover, the present data indicate that MPEP does not substitute for the stimulus effects of ethanol, indicating minimal coabuse liability. Further examination of potential interactions between ethanol's physiological and behavioral effects and the mGlu5 receptor system may indeed reveal the mGluR5 system to be a novel and effective therapeutic target in the treatment of alcoholism.
References
Akk G, Steinbach JH (2000). Activation and block of recombinant GABA(A) receptors by pentobarbitone: a single-channel study. Br J Pharmacol 130: 249–258.
Aschner M, Mutkus L, Allen JW (2001). Aspartate and glutamate transport in acutely and chronically ethanol exposed neonatal rat primary astrocyte cultures. Neurotoxicology 22: 601–605.
Ator NA, Grant KA, Purdy RH, Paul SM, Griffiths RR (1993). Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol 241: 237–243.
Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ (2000). Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci 20: 7871–7879.
Bäckström P, Bachteler D, Koch S, Hyytiä P, Spanagel R, National Public Health Institute HFdz-md (2004). mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 29: 921–928.
Barry M (1991). Interactions of drugs with alcohol. Practitioner 235: 270–272.
Benquet P, Gee CE, Gerber U (2002). Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci 22: 9679–9686.
Besheer J, Cox AA, Hodge CW (2003). Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res 27: 450–456.
Bordi F, Ugolini A (1999). Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol 59: 55–79.
Bormann J (1988). Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci 11: 112–116.
Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S et al (2001). Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci 4: 873–874.
Costa ET, Savage DD, Valenzuela CF (2000). A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res 24: 706–715.
de Novellis V, Marabese I, Palazzo E, Rossi F, Berrino L, Rodella L et al (2003). Group I metabotropic glutamate receptors modulate glutamate and gamma-aminobutyric acid release in the periaqueductal grey of rats. Eur J Pharmacol 462: 73–81.
Diaz-Cabiale Z, Vivo M, Del Arco A, O'Connor WT, Harte MK, Muller CE et al (2002). Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci Lett 324: 154–158.
Dodd PR, Beckmann AM, Davidson MS, Wilce PA (2000). Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int 37: 509–533.
Evans EB, Balster RL (1991). CNS depressant effects of volatile organic solvents. Neurosci Biobehav Rev 15: 233–241.
Fuchs K, Mohler H, Sieghart W (1988). Various proteins from rat brain, specifically and irreversibly labeled by [3H]flunitrazepam, are distinct alpha-subunits of the GABA–benzodiazepine receptor complex. Neurosci Lett 90: 314–319.
Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I et al (1999). 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38: 1493–1503.
Gereau RWT, Conn PJ (1995). Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci 15: 6879–6889.
Gonzales RA, Theiss C, Crews FT (1986). Effects of ethanol on stimulated inositol phospholipid hydrolysis in rat brain. J Pharmacol Exp Ther 237: 92–98.
Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH (1996). Ethanol-like discriminative stimulus effects of the neurosteroid 3 alpha-hydroxy-5 alpha-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology 124: 340–346.
Grant KA, Azarov A, Shively CA, Purdy RH (1997). Discriminative stimulus effects of ethanol and 3 alpha-hydroxy-5 alpha-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis). Psychopharmacology 130: 59–68.
Grant KA, Colombo G (1993). Pharmacological analysis of the mixed discriminative stimulus effects of ethanol. Alcohol Alcoholism Suppl 2: 445–449.
Grant KA, Lovinger DM (1995). Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci 3: 155–164.
Green-Jordan K, Grant KA (2000). Modulation of the ethanol-like discriminative stimulus effects of diazepam and phencyclidine by L-type voltage-gated calcium-channel ligands in rats. Psychopharmacology 149: 84–92.
Gruol DL, Parsons KL, DiJulio N (1997). Acute ethanol alters calcium signals elicited by glutamate receptor agonists and K+ depolarization in cultured cerebellar Purkinje neurons. Brain Res 773: 82–89.
Härtig W, Brauer K, Fritschy JM, Bruckner G, Bigl V (1995). Regional and cellular expression sites of the alpha 1 subunit of GABAA receptors in the rat basal forebrain: a cytochemical study with glutamic acid decarboxylase, choline acetyltransferase, calcium-binding proteins and nitric oxide synthase as second markers. Brain Res 692: 215–226.
Hodge CW, Cox AA (1998). The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology 139: 95–107.
Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF et al (1999). Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci 2: 997–1002.
Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK (2001). Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res 25: 1441–1447.
Hoffpauir BK, Gleason EL (2002). Activation of mGluR5 modulates GABA(A) receptor function in retinal amacrine cells. J Neurophysiol 88: 1766–1776.
Jarbe TU, McMillan DE (1983). Interaction of the discriminative stimulus properties of diazepam and ethanol in pigeons. Pharmacol Biochem Behav 18: 73–80.
June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC et al (2003). The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology 28: 2124–2137.
Kostowski W, Bienkowski P (1999). Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol 17: 63–80.
Krasowski MD, Harrison NL (1999). General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci 55: 1278–1303.
Littleton JM, Lovinger D, Liljequist S, Ticku R, Matsumoto I, Barron S (2001). Role of polyamines and NMDA receptors in ethanol dependence and withdrawal. Alcohol Clin Exp Res 25: 132S–136S.
Lu XY, Ghasemzadeh MB, Kalivas PW (1999). Expression of glutamate receptor subunit/subtype messenger RNAS for NMDAR1, GLuR1, GLuR2 and mGLuR5 by accumbal projection neurons. Brain Res Mol Brain Res 63: 287–296.
McGeehan AJ, Olive MF (2003). The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse 47: 240–242.
McKernan RM, Whiting PJ (1996). Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19: 139–143.
Mihic SJ (1999). Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int 35: 115–123.
Minami K, Gereau RWT, Minami M, Heinemann SF, Harris RA (1998). Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol Pharmacol 53: 148–156.
Mohler H, Benke D, Benson J, Luscher B, Rudolph U, Fritschy JM (1997). Diversity in structure, pharmacology, and regulation of GABA(A) receptors. Bowery NG, Enna SJ (ed). The GABA Receptors. Humana Press Inc.: Totowa, NJ, pp 11–36.
Netzeband JG, Gruol DL (1995). Modulatory effects of acute ethanol on metabotropic glutamate responses in cultured Purkinje neurons. Brain Res 688: 105–113.
Netzeband JG, Schneeloch JR, Trotter C, Caguioa-Aquino JN, Gruol DL (2002). Chronic ethanol treatment and withdrawal alter ACPD-evoked calcium signals in developing Purkinje neurons. Alcohol Clin Exp Res 26: 386–393.
O'Malley KL, Jong YJ, Gonchar Y, Burkhalter A, Romano C (2003). Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem 278: 28210–28219.
Pintor A, Pezzola A, Reggio R, Quarta D, Popoli P (2000). The mGlu5 receptor agonist CHPG stimulates striatal glutamate release: possible involvement of A2A receptors. Neuroreport 11: 3611–3614.
Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000). GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850.
Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D et al (2001). Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 106: 579–587.
Poisik OV, Mannaioni G, Traynelis S, Smith Y, Conn PJ (2003). Distinct functional roles of the metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. J Neurosci 23: 122–130.
Popik P, Wrobel M (2002). Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology 43: 1210–1217.
Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW (1995). Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 355: 455–469.
Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W et al (2001). Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol 433: 526–549.
Serafini R, Bracamontes J, Steinbach JH (2000). Structural domains of the human GABAA receptor 3 subunit involved in the actions of pentobarbital. J Physiol 524: 649–676.
Sharko AC, Iller K, Koening H, Lau K, Ou CJ, Olive MF et al (2002). Involvement of metabotropic glutamate receptor subtype 5 (mGlu5) in alcohol self-administration. Alcohol Clin Exp Res Suppl 26: 112A.
Shelton KL, Grant KA (2002). Discriminative stimulus effects of ethanol in C57BL/6J and DBA/2J inbred mice. Alcohol Clin Exp Res 26: 747–757.
Simonyi A, Zhang JP, Sun AY, Sun GY (1996). Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. Neuroreport 7: 2115–2118.
Skerritt JH, Macdonald RL (1984). Benzodiazepine receptor ligand actions on GABA responses. Benzodiazepines, CL 218872, zopiclone. Eur J Pharmacol 101: 127–134.
Spooren WP, Gasparini F, Bergmann R, Kuhn R (2000). Effects of the prototypical mGlu(5) receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine on rotarod, locomotor activity and rotational responses in unilateral 6-OHDA-lesioned rats. Europ J Pharmacol 406: 403–410.
Spooren WP, Gasparini F, Salt TE, Kuhn R (2001). Novel allosteric antagonists shed light on mglu(5) receptors and CNS disorders. Trends Pharmacol Sci 22: 331–337.
Steinbach JH, Akk G (2001). Modulation of GABA(A) receptor channel gating by pentobarbital. J Physiol 537: 715–733.
Stephenson FA, Duggan MJ, Pollard S (1990). The gamma 2 subunit is an integral component of the gamma-aminobutyric acidA receptor but the alpha 1 polypeptide is the principal site of the agonist benzodiazepine photoaffinity labeling reaction. J Biol Chem 265: 21160–21165.
Stolerman I (1992). Drugs of abuse: behavioural principles, methods and terms. Trends in Pharmacol Sci 13: 170–176.
Study RE, Barker JL (1981). Diazepam and (−−)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci USA 78: 7180–7184.
Sung KW, Choi S, Lovinger DM (2001). Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol 86: 2405–2412.
Tabakoff B, Hoffman PL (1993). Ethanol, sedative hypnotics, and glutamate receptor function in brain and cultured cells. Behav Genet 23: 231–236.
Weight FF, Peoples RW, Wright JM, Lovinger DM, White G (1993). Ethanol action on excitatory amino acid activated ion channels. Alcohol Alcoholism Suppl 2: 353–358.
Woodward JJ (1999). Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochem Int 35: 107–113.
Acknowledgements
This work was supported by Grants AA09981 and AA011605 from the National Institute on Alcohol Abuse and Alcoholism to CWH. We would like to thank Dr Jean-Marc Fritschy for the gift of the GABAA α1 antibody, Drs John J Lemasters and Jae Sung Kim from the Bowles Center for Alcohol Studies Imaging core, and Mary Beth Wilkie for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Besheer, J., Hodge, C. Pharmacological and Anatomical Evidence for an Interaction Between mGluR5- and GABAA α1-Containing Receptors in the Discriminative Stimulus Effects of Ethanol. Neuropsychopharmacol 30, 747–757 (2005). https://doi.org/10.1038/sj.npp.1300616
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300616
Keywords
This article is cited by
-
From bench to bedside: The mGluR5 system in people with and without Autism Spectrum Disorder and animal model systems
Translational Psychiatry (2022)
-
Interaction of Glutamate Receptors and GABA Neurons in the Central Nervous System
Neuroscience and Behavioral Physiology (2017)
-
Stress Hormone Exposure Reduces mGluR5 Expression in the Nucleus Accumbens: Functional Implications for Interoceptive Sensitivity to Alcohol
Neuropsychopharmacology (2014)
-
The effects of repeated corticosterone exposure on the interoceptive effects of alcohol in rats
Psychopharmacology (2012)
-
Activation of Group II Metabotropic Glutamate Receptors Inhibits the Discriminative Stimulus Effects of Alcohol via Selective Activity Within the Amygdala
Neuropsychopharmacology (2011)