Abstract
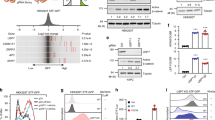
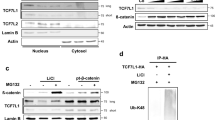
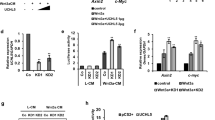
β-Catenin-mediated Wnt signaling is essential in embryonic development and in adult tissues. Recent studies have demonstrated that Axin not only plays an important inhibitory role in coordinating β-catenin degradation, but is itself degraded by the low-density-lipoprotein receptor-related protein (LRP)5/6 Wnt co-receptor. Here, we demonstrate that the endocytic adaptor molecule Disabled-2 (Dab2), which we have previously demonstrated to act as an inhibitor of β-catenin signaling, interacts with Axin and prevents its interaction with and degradation by the LRP5 co-receptor, thereby increasing its half-life and stabilization. Dab2 levels induced during retinoic acid-induced differentiation of F9, or during transforming growth factor-β-induced epithelial–mesenchymal transdifferentiation of mouse mammary epithelial cells result in the stabilization of Axin and concomitant inhibition of β-catenin signaling. Ectopic expression of Dab2 in F9 cells as well as in transformed cell lines results in increased Axin expression and attenuation of Wnt-mediated signaling. We conclude that Dab2 may play an important role in the maintenance of the differentiated state and restrain Wnt-mediated proliferation through its association with and modulation of Axin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bilic J, Huang Y-L, Davidson G, Zimmermann T, Cruciat C-M, Bienz M et al. (2007). Wnt induces LRP6 signalosomes and promotes Dishevelled-dependent LRP6 phosphorylation. Science 316: 1619–1622.
Cadigan KM, Liu YI . (2005). Wnt signaling: complexity at the surface. J Cell Sci 119: 395–402.
Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M . (2002). The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419: 726–729.
Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE et al. (2003). Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301: 1391–1394.
Cliffe A, Hamada F, Bienz M . (2003). A role of dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol 13: 960–966.
Daniel JM, Reynolds AB . (1995). The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol 15: 4819–4824.
Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C et al. (1999). Domains of Axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol 145: 741–756.
Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y et al. (2001). Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol 21: 5132–5141.
Gregorieff A, Clevers H . (2005). Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 19: 877–890.
He X, Semenov M, Tamai K, Zeng X . (2004). LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131: 1663–1677.
Herber B, Truss M, Beato M, Müller R . (1994). Inducible regulatory elements in the human cyclin D1 promoter. Oncogene 9: 1295–1304.
Hocevar BA, Mou F, Rennolds JL, Morris SM, Cooper JA, Howe PH . (2003). Interactions between disabled-2 (Dab2) and the Wnt signaling pathway. EMBO J 22: 3084–3094.
Hocevar BA, Smine A, Xu X-X, Howe PH . (2001). The adaptor molecule Disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J 20: 2789–2801.
Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF . (1997). catenin mutations in cell lines established from colorectal cancers. Proc Natl Acad Sci USA 94: 10330–10334.
Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F . (2002). Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183.
Kakimura DM, Cooper JA . (2006). Clathrin interaction and subcellular localization of Ce-Dab-1, an adaptor for protein secretion in Caenorhabditis elegans. Traffic 7: 324–336.
Kleeff J, Huang Y, Mok SC, Zimmermann A, Friess H, Buchler MW . (2002). Down-regulation of DOC-2 in colorectal cancer points to its role as a tumor suppressor in this malignancy. Dis Colon Rectum 45: 1242–1245.
Lee E, Salic A, Kruger R, Heinrich R, Kirchner MW . (2003). The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol 1: 116–132.
Lickert H, Cox B, Wehrle C, Taketo MM, Kemler R, Rossant J . (2005). Dissecting Wnt/β-catenin signaling during gastrulation using RNA interference in mouse embryos. Development 132: 2599–2609.
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y et al. (2002). Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847.
Mishra SK, Keyel PA, Hawryluk J, Agostinelli NR, Watkins SC, Traub LM . (2002). Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J 21: 4915–4926.
Morin PJ, Vogelstein B, Kinzler KW . (1996). Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci USA 93: 7950–7954.
Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP et al. (2002). Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic 3: 331–341.
Morris SM, Cooper JA . (2001). Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2: 111–123.
Polakis P . (2002). Casein kinase I: a Wnt'er of disconnect. Curr Biol 12: R499–R501.
Prunier C, Hocevar BH, Howe PH . (2004). Wnt signaling: physiology and pathology. Growth Factors 22: 141–150.
Prunier C, Howe PH . (2005). Disabled-2 (Dab2) is required for transforming growth factor β-induced epithelial to mesenchymal transition (EMT). J Biol Chem 280: 17540–17548.
Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S . (1998). Cytoskeletal reorganization by soluble Wnt-3a protein signaling. Genes Cells 3: 659–670.
Shibamoto S, Winer J, Williams M, Polakis P . (2004). A blockade in Wnt signaling is activated following the differentiation of F9 teratocarcinoma cells. Exp Cell Res 292: 11–20.
Smith ER, Capochichi CD, He J, Smedberg JL, Yang DH, Prowse AH et al. (2001). Disabled-2 mediates c-Fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J Biol Chem 276: 47303–47310.
Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z et al. (2004). A mechanism for Wnt coreceptor activation. Mol Cell 13: 149–156.
Tolwinski NS, Wieschaus E . (2004). Rethinking Wnt signaling. Trends Genet 20: 177–181.
Wiles MV . (1988). Isolation of differentially expressed human cDNA clones: similarities between mouse and human embryonal carcinoma cell differentiation. Development 104: 403–413.
Willert K, Shibamoto S, Nusse R . (1999). Wnt-induced dephosphorylation of Axin releases beta-catenin from the Axin complex. Genes Dev 13: 1768–1773.
Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D et al. (2003). Direct binding of the PDX domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell 12: 1251–1260.
Xu XX, Yang W, Jackowski S, Rock CO . (1995). Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J Biol Chem 270: 14184–14191.
Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A . (1999). Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J Biol Chem 274: 10681–10684.
Yamamoto H, Komekado H, Kikuchi A . (2006). Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev Cell 11: 213–223.
Yu A, Rual J-F, Tamai K, Harada Y, Vidal M, He X et al. (2007). Association of dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell 12: 129–141.
Yun M, Keshvara L, Park CG, Zhang YM, Dickerson JB, Zheng J et al. (2003). Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J Biol Chem 278: 36572–36581.
Zeng X, Tamai K, Doble B, Shita L, Huang H, Habas R et al. (2005). A dual-mechanism for Wnt co-receptor phosphorylation and activation. Nature 438: 873–877.
Acknowledgements
We thank Dr S Takada for the generous provision of control Wnt-3A-producing mouse L-cells and Drs Furuhashi, Wu, Zeng and He for generous provision of the Myc–Axin, Flag-tagged LRP5 plasmids and LRP5/6 antibody, respectively. We also thank Dr Gary Wildey for helpful discussion and also thank Dr S Ledbetter at Genzyme Inc. for generous provision of TGFβ This work was supported by Grants CA55536 and CA80095 from the National Cancer Institute to PHH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Jiang, Y., Prunier, C. & Howe, P. The inhibitory effects of Disabled-2 (Dab2) on Wnt signaling are mediated through Axin. Oncogene 27, 1865–1875 (2008). https://doi.org/10.1038/sj.onc.1210829
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210829
Keywords
This article is cited by
-
RepID represses megakaryocytic differentiation by recruiting CRL4A-JARID1A at DAB2 promoter
Cell Communication and Signaling (2023)
-
Disabled-2: a positive regulator of the early differentiation of myoblasts
Cell and Tissue Research (2020)
-
X-ray irradiation induced Disabled-2 gene promoter de-methylation enhances radiosensitivity of non-small-cell lung carcinoma cells
Journal of Experimental & Clinical Cancer Research (2018)
-
Pathways on demand: automated reconstruction of human signaling networks
npj Systems Biology and Applications (2016)
-
Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk
Nature Communications (2014)