Abstract
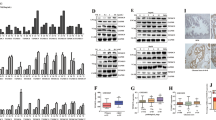
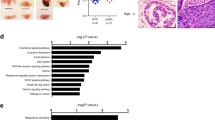
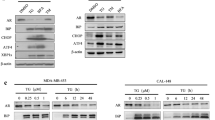
Human WWOX gene encodes a proapoptotic WW domain-containing oxidoreductase WOX1 (also named WWOX, FOR2 or WWOXv1). Apoptotic and stress stimuli activate WOX1 via Tyr33 phosphorylation and nuclear translocation. WOX1 possesses a tetrad NSYK motif in the C-terminal short-chain alcohol dehydrogenase/reductase (SDR) domain, which may bind estrogen and androgen. Here, we determined that 17β-estradiol (E2) activated WOX1, p53 and ERK in COS7 fibroblasts, primary lung epithelial cells, and androgen receptor (AR)-negative prostate DU145 cells, but not in estrogen receptor (ER)-positive breast MCF7 cells. Androgen also activated WOX1 in the AR-negative DU145 cells. These observations suggest that sex hormone-mediated Tyr33 phosphorylation and nuclear translocation of WOX1 is independent of ER and AR. Stress stimuli increase physical binding of p53 with WOX1 in vivo. We determined here that E2 increased the formation of p53/WOX1 complex and their nuclear translocation in COS7 cells; however, nuclear translocation of this complex could not occur in MCF7 cells. By immunohistochemistry, we determined that progression of prostate from normal to hyperplasia, cancerous and metastatic stages positively correlate with upregulation and activation of WOX1 and WOX2 (FOR1/WWOXv2). In contrast, breast cancer development to a premetastatic state is associated with upregulation and Tyr33 phosphorylation of cytosolic WOX1 and WOX2, followed by significant downregulation or absent expression during metastasis. These Tyr33-phosphorylated proteins are mostly located in the mitochondria without translocating to the nuclei, which is comparable to those findings in cultured breast cancer cells. Together, sex steroid hormone-induced activation of WOX1 and WOX2 is independent of ER and AR, and this activation positively correlates with cancerous progression of prostate and breast to a premetastatic state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K and Croce CM . (2004). Proc. Natl. Acad. Sci. USA, 101, 4401–4406.
Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA and Aldaz CM . (2000). Cancer Res., 60, 2140–2145.
Bronzert DA, Hochberg RB and Lippman ME . (1982). Endocrinology, 110, 2177–2182.
Burchardt M, Burchardt T, Shabsigh A, Ghafar M, Chen MW, Anastasiadis A, de la Taille A, Kiss A and Buttyan R . (2001). Prostate, 48, 225–230.
Caligo MA, Polidoro L, Ghimenti C, Campani D, Cecchetti D and Bevilacqua G . (1998). Int. J. Oncol., 13, 177–182.
Chang N-S . (2002a). Int. J. Mol. Med., 9, 19–24 (review).
Chang N-S . (2002b). BMC Cell Biol., 3, 8.
Chang N-S, Doherty J and Ensign A . (2003a). J. Biol. Chem., 278, 9195–9202.
Chang N-S, Doherty J, Ensign A, Lewis J, Heath J, Schultz L, Chen ST and Oppermann U . (2003b). Biochem. Pharmacol., 66, 1347–1354 (review).
Chang N-S, Pratt N, Heath J, Schultz L, Sleve D, Carey GB and Zevotek N . (2001). J. Biol. Chem., 276, 3361–3370.
Chen ST, Chuang JI, Cheng CL, Hsu LJ and Chang N-S . (2004a). Neuroscience in press.
Chen ST, Chuang JI, Wang JP, Tsai MS, Li H and Chang N-S . (2004b). Neuroscience, 124, 831–839.
Coezy E, Borgna JL and Rochefort H . (1982). Cancer Res., 42, 317–323.
Driouch K, Prydz H, Monese R, Johansen H, Lidereau R and Frengen E . (2002). Oncogene, 21, 1832–1840.
Elo JP, Harkonen P, Kyllonen AP, Lukkarinen O and Vihko P . (1999). Br. J. Cancer, 79, 156–160.
Filling C, Berndt KD, Benach J, Knapp S, Prozorovski T, Nordling E, Ladenstein R, Jornvall H and Oppermann U . (2002). J. Biol. Chem., 277, 25677–25684.
Filling C, Wu X, Shafqat N, Hult M, Martensson E, Shafqat J and Oppermann UC . (2001). Mol. Cell. Endocrinol., 171, 99–101.
Gaitonde SV, Riley JR, Qiao D and Martinez JD . (2000). Oncogene, 19, 4042–4049.
Gangolli EA, Conneely OM and O'Malley BW . (1997). J. Steroid Biochem. Mol. Biol., 61, 1–9.
Garcia-Segura LM, Azcoitia I and DonCarlos LL . (2001). Prog. Neurobiol., 63, 29–60 (review).
Gartel AL, Feliciano C and Tyner AL . (2003). Oncol Res., 13, 405–408.
Gompel A, Somai S, Chaouat M, Kazem A, Kloosterboer HJ, Beusman I., Forgez P, Mimoun M and Rostene W . (2000). Steroids, 65, 593–598 (review).
Green PS, Bishop J and Simpkins JW . (1997). J. Neurosci., 17, 511–515.
Gridley KE, Green PS and Simpkins JW . (1998). Mol. Pharmacol., 54, 874–880.
Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P and Huebner K . (2004). Cancer, 100, 1605–1614.
He XY, Merz G, Yang YZ, Mehta P, Schulz H and Yang SY . (2001). Eur. J. Biochem., 268, 4899–4907.
Isaacs WB, Carter BS and Ewing CM . (1991). Cancer Res., 51, 4716–4720.
Kallberg Y, Oppermann U, Jornvall H and Persson B . (2002). Eur. J. Biochem., 269, 4409–4417.
Keshamouni VG, Mattingly RR and Reddy KB . (2002). J. Biol. Chem., 277, 22558–22565.
Lau KM, LaSpina M, Long J and Ho SM . (2000). Cancer Res., 60, 3175–3182.
Li C, Berx G, Larsson C, Auer G, Aspenblad U, Pan Y, Sundelin B, Ekman P, Nordenskjold M, van Roy F and Bergerheim US . (1999). Genes Chromosomes Cancer, 24, 175–182.
Linja MJ, Savinainen KJ, Tammela TL, Isola JJ and Visakorpi T . (2003). Prostate, 55, 180–186.
Lokeshwar V and Rubinowicz D . (1999). Prostate Cancer PD, 2, S21.
Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, Nadji M and Lokeshwar BL . (2001). J. Biol. Chem., 276, 11922–11932.
Ludes-Meyers JH, Kil H, Bednarek AK, Drake J, Bedford MT and Aldaz CM . (2004). Oncogene, 23, 5049–5055.
Marker PC, Donjacour AA, Dahiya R and Cunha GR . (2003). Dev. Biol., 253, 165–174 (review).
Nesslinger NJ, Shi XB and deVere White RW . (2003). Cancer Res., 63, 2228–2233.
Patel S, Turner PR, Stubberfield C, Barry E, Rohlff CR, Stamps A, McKenzie E, Young K, Tyson K, Terrett J, Box G, Eccles S and Page MJ . (2002). Int. J. Cancer, 97, 416–424.
Richards RI . (2001). Trends Genet., 17, 339–345 (review).
Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E and Richards RI . (2000). Hum. Mol. Genet., 9, 1651–1663.
Setalo Jr G, Singh M, Guan X and Toran-Allerand CD . (2002). J. Neurobiol., 50, 1–12.
Song RX and Santen RJ . (2003). Apoptosis, 8, 55–60 (review).
Sze CI, Su M, Pugazhenthi S, Jambal P, Hsu LJ, Heath J, Schultz L and Chang N-S . (2004). J Biol Chem., 279, 30498–30506.
Toillon RA, Chopin V, Jouy N, Fauquette W, Boilly B and Le Bourhis X . (2002). Breast Cancer Res Treat., 71, 269–280.
Watanabe A, Hippo Y, Taniguchi H, Iwanari H, Yashiro M, Hirakawa K, Kodama T and Aburatani H . (2003). Cancer Res., 63, 8629–8633.
Watters JJ, Campbell JS, Cunningham MJ, Krebs EG and Dorsa DM . (1997). Endocrinology, 138, 4030–4033.
Wise PM . (2002). Trends Endocrinol Metab., 13, 229–230 (review).
Wolff A, Technau A, Ihling C, Technau-Ihling K, Erber R, Bosch FX and Brandner G . (2001). Oncogene, 20, 1307–1317.
Wollin M, FitzGerald TJ, Santucci MA, Menon M, Longcope C, Reale F, Carlson J, Sakakeeny MA and Greenberger JS . (1989). Radiother. Oncol., 15, 285–293.
Xu Y, Song J, Berelowitz M and Bruno JF . (1996). Endocrinology, 137, 5634–5640.
Acknowledgements
Research was supported by the Department of Defense (DAMD17-03-1-0736) to NSC. We thank Ms Terri Zimmer for antibody production in rabbits, and Dr Robert I Richards of the University of Adelaide, Australia, in contributing to the production of antibodies against WOX1/FOR2 and WOX2/FOR1. LJH is currently a visiting scientist from the National Cheng Kung University Medical College, Taiwan, and supported by the Ministry of Education, Taiwan, Republic of China (Grant 91-B-FA09-1-4). JL was a Guthrie Scholar from the Wilkes University, Wilkes-Barre, PA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information accompanies the paper on Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Chang, NS., Schultz, L., Hsu, LJ. et al. 17β-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 24, 714–723 (2005). https://doi.org/10.1038/sj.onc.1208124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208124
Keywords
This article is cited by
-
WWOX and metabolic regulation in normal and pathological conditions
Journal of Molecular Medicine (2022)
-
TGFα-EGFR pathway in breast carcinogenesis, association with WWOX expression and estrogen activation
Journal of Applied Genetics (2022)
-
Normal cells repel WWOX-negative or -dysfunctional cancer cells via WWOX cell surface epitope 286-299
Communications Biology (2021)
-
A p53/TIAF1/WWOX triad exerts cancer suppression but may cause brain protein aggregation due to p53/WWOX functional antagonism
Cell Communication and Signaling (2019)
-
Strategies by which WWOX-deficient metastatic cancer cells utilize to survive via dodging, compromising, and causing damage to WWOX-positive normal microenvironment
Cell Death Discovery (2019)