Abstract
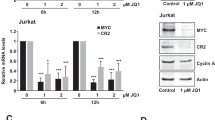
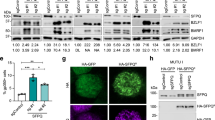
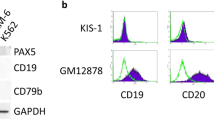
Expression of EBNA3C is essential for the immortalization of B cells by EBV in vitro and, in co-operation with activated ras, EBNA3C has oncogenic activity in primary rodent fibroblasts. This suggested that this viral oncoprotein might disrupt the cyclin/CDK-pRb-E2F pathway, which regulates cell cycle progression at the restriction point (R-point) in G1 of the proliferation cycle. An assay was established in which transfected EBNA3C-positive cells could be sorted and simultaneously analysed for their distribution in the cell cycle. This revealed that in NIH3T3 fibroblasts compelled to arrest by serum-withdrawal, EBNA3C induces nuclear division that is often divorced from cytokinesis and so produces bi- and multinucleated cells. This was confirmed using the ecdysone-inducible system for expression of EBNA3C in human U2OS cells and by microinjection of expression vectors into NIH3T3 and U2OS. Further analysis revealed that in the inducible system, EBNA3C expression inhibits the accumulation of p27KIP1 but not the dephosphorylation of pRb. Experiments using the microtubule destabilizing drug nocodazole, showed that EBNA3C could abrogate the mitotic spindle checkpoint.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Babcock GJ, Decker LL, Volk M and Thorley-Lawson DA. . 1998 Immunity 9: 395–404.
Bain M, Watson R, Farrell PJ and Allday MJ. . 1996 J. Virol. 70: 2481–2489.
Coats S, Flanagan WM, Nourse J and Roberts JM. . 1996 Science 272: 877–880.
Crook T, Parker GA, Rozyka M, Crossland S and Allday MJ. . 1998 Oncogene 16: 1429–1441.
DePinho RA. . 1998 Nature 391: 533–536.
Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM, Barnes DM, Shousha S, O'Hare M and Lu X. . 1997 Proc. Natl. Acad. Sci. USA 94: 6380–6385.
Hengst L and Reed SI. . 1996 Science 271: 1861–1864.
Jin D-Y, Spencer F and Jeang K-T. . 1998 Cell 93: 81–91.
Kempkes B, Pich D, Zeidler R, Sugden B and Hammerschmidt W. . 1995 J. Virol. 69: 231–238.
Kieff E. . 1996 In: Fields Virology, 3rd edition, Fields BN, Knipe DM, Howley PM, et al. (eds).. Lippincott-Raven Publishers: Philadelphia.
Kung AL, Sherwood SW, Schimke RT. . 1990 Proc. Natl. Acad. Sci. USA 87: 9553–9557.
Morgan DO. . 1999 Nature Cell Biol. 1: 47–53.
Orr-Weaver TL and Weinberg RA. . 1998 Nature 392: 223–224.
Pagano M, Tam S, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF and Rolfe M. . 1995 Science 269: 682–685.
Parker GA, Crook T, Bain M, Sara EA, Farrell PJ and Allday MJ. . 1996 Oncogene 13: 2541–2549.
Radkov SA, Bain M, Farrell PJ, West M, Rowe M and Allday MJ. . 1997 J. Virol. 71: 8852–8862.
Radkov SA, Touitou R, Brehm A, Rowe M, West M, Kouzarides T and Allday M. . 1999 J. Virol. 73: 5688–5697.
Rickinson A and Kieff E. . 1996 In: Fields Virology, 3rd edition, Fields BN, Knipe DM, Howley PM, et al. (eds).. Lippincott-Raven Publishers: Philadelphia.
Sherr CJ and Roberts J. . 1995 Genes Dev. 9: 1149–1163.
Shimizu E, Coxon A, Otterson, GA, Steinberg SM, Kratzke RA, et al. 1994 Oncogene 9: 2441–2448.
Taylor SS and McKeon F. . 1998 Cell 89: 727–735.
Tomkinson B, Robertson E and Kieff E. . 1993 J. Virol. 67: 2014–2025.
Waltzer L, Perricaudet M, Sergeant A and Manet E. . 1996 J. Virol. 70: 5909–5915.
Weinberg RA. . 1995 Cell 81: 323–330.
Acknowledgements
Thanks to Martin Rowe (Cardiff) for antiEBNA3C mAbs and Xin Lu (LICR) for antiPCNA and p27 mAbs. We are indebted to Roger Watson for his insightful comments that led to the initial discovery of the polyploid cells. We are also grateful to Anne Ridley (LICR, University College, London) for her help in initiating the microinjection study and Gareth Inman for helpful comments on the manuscript. This research was supported by the Wellcome Trust through a project grant to MJA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parker, G., Touitou, R. & Allday, M. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene 19, 700–709 (2000). https://doi.org/10.1038/sj.onc.1203327
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203327
Keywords
This article is cited by
-
Epstein–Barr virus particles induce centrosome amplification and chromosomal instability
Nature Communications (2017)
-
Post-transplant lymphoproliferative disorders
Nature Reviews Disease Primers (2016)
-
Epstein–Barr virus BZLF1 protein impairs accumulation of host DNA damage proteins at damage sites in response to DNA damage
Laboratory Investigation (2015)
-
The DNA damage response in viral-induced cellular transformation
British Journal of Cancer (2012)