Abstract
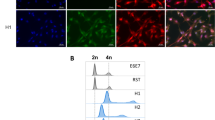
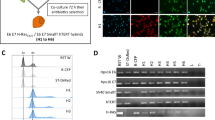
The highly metastatic amelanotic C8161 human melanoma line was found to exhibit complete dominance of its undifferentiated and metastatic phenotype in multiple somatic cell hybridization studies designed to bypass the presence of potential tumor suppressor genes. In a three armed approach involving somatic cell fusions of C8161 with recipient lines of greater differentiation, different lineage, and different tumorigenicity status, the metastatic and undifferentiated phenotype of C8161 was promiscuously dominant. In somatic cell hybrids produced between the C8161 and a group of non-metastatic human melanoma lines which exhibited melanocyte differentiation markers including S100, HMB-45, NKI/C3, and melanin, the fusions were uniformly metastatic and undifferentiated. In somatic cell hybrids of C8161 and MCF-7 the fusions exhibited an estrogen independent and unresponsive, estrogen receptor (ER) negative, and highly metastatic phenotype. In fusions between C8161 and HMS-1, an immortalized `benign' human myoepithelial line which produced an abundant extracellular matrix (ECM) and high levels of protease and angiogenic inhibitors including maspin, tissue inhibitor of metalloproteinase-1 (TIMP-1), α1-antitrypsin (α1-AT), protease nexin II (PN-II), thrombospondin-1 and soluble basic fibroblast growth factor (bFGF) receptors, the hybrids showed complete absence of matrix, absent maspin expression, markedly decreased protease inhibitor and angiogenic inhibitor production, high levels of proteases and angiogenic factors, and a highly metastatic phenotype. In our somatic cell fusions, the human-human hybrids represented true and complete fusions and not hybrid clones selected for by loss of dominant-acting growth suppressor genes. This finding was supported by detailed comparative genomic hybridization (CGH) studies, Q-banding karyotype analysis, and autofusions of representative clones. The purposeful creation of inherently unstable human-murine fusions between C8161 and B16-F1 where loss of putative suppressor loci would be expected, resulted in fusions exhibiting decreased growth and non-metastatic behavior with progressive chromosomal loss. Neither p53, nm23, DNA methyltransferase, activated ras, fibroblast growth factor-4 (FGF-4), or epidermal growth factor receptor (EGFR) mediated the acquisition of the metastatic or undifferentiated phenotype within the C8161-human fusions. These studies are the first studies ever to successfully transfer the complete metastatic phenotype by somatic cell fusion and support the presence of a new high level regulatory pathway(s) involving dominant trans-acting factors which act pleiotropically to regulate an undifferentiated and highly metastatic phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barsky, S., Sternlicht, M., Safarians, S. et al. Evidence of a dominant transcriptional pathway which regulates an undifferentiated and complete metastatic phenotype. Oncogene 15, 2077–2091 (1997). https://doi.org/10.1038/sj.onc.1201379
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1201379
Keywords
This article is cited by
-
Short and long-term barriers and facilitators of skin self-examination among individuals diagnosed with melanoma
BMC Cancer (2020)
-
Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma
Oncogene (2002)
-
Human breast carcinoma desmoplasia is PDGF initiated
Oncogene (2000)