Abstract
Background:
Somatic cutaneous small sensory fiber neuropathy (SSFN) can be an early manifestation of impaired glucose tolerance and diabetes mellitus and/or insulin resistance among obese subjects and is often associated with pain, wound occurrence and impaired wound healing. It is yet unclear as to whether SSFN is prevalent among obese individuals without glucose and/or insulin dysregulation despite abundant evidence of delayed wound healing.
Objective:
To observe whether there is hypofunctioning of stimulated capsaicin-sensitive cutaneous nerves (small sensory fibers) in obese subjects with/without hyperglycemia and hyperinsulinemia.
Design, setting and participants:
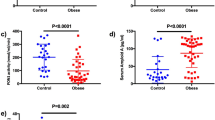
Fifty-eight morbidly obese and 15 lean subjects were recruited for small fiber testing of the forearm in a cross-sectional study. Hyperglycemia was observed in 35 obese subjects. Of 25 obese subjects, hyperinsulinemia was noted in 15, 14 of which were hyperglycemic. No subjects demonstrated symptoms/signs of neuropathy over the hairy skin of the forearm. In fact, a neurological examination revealed that 37 subjects were asymptomatic in the legs and only four complained of a neuropathic pain in the foot. Virtually all subjects were exposed to a set of capsaicin-sensitive tests and measures which were identified by capsaicin desensitization procedures. These tests, conducted while in a supine position in bed at the Banner Good Samaritan Medical Center, Phoenix, examined the two principle roles of cutaneous SSFs, namely conveying pain signals to the CNS and controlling local neurogenic vasodilatation (flare; axon-reflex).
Main outcome measures:
Heat-induced pain was assessed by verbal reports of sensation after accommodation and heat-, capsaicin-, and transcutneous stimulation- induced blood flow was measured by laser Doppler flowmetry with probes placed at the site of stimulation and 1 cm remote from the site, the latter to evaluate flare latency and intensity of flare.
Results:
Significant depression of pain and flare responses were observed in the obese subjects in all but one test. Decreased pain and flare responses were noted in all subjects without hyperglycemia and hyperinsulinemia. Age negatively correlated with capsaicin-induced flare in both the obese and normal groups.
Conclusion:
SSFN was prevalent in the cohort of morbidly obese subjects in a skin area without neurological symptoms or signs and in subjects with/without hyperglycemia and hyperinsulinemia. SSFN may be a serious factor in observations of impaired wound healing among obese subjects, a particularly worrisome problem in an obese aging population given the propensity for small fiber impairment in aging subjects. Small fiber impairment in the younger obese population may signal an early aging phenomenon.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Beller GA . President's page: the epidemic of type II diabetes and obesity in the US: cause for alarm. J Am Coll Cardiol 2000; 36: 2348–2350.
Ochoa J, Mair W . The normal sural nerve in man. Acta Neuropathol 1969; 13: 197–216.
Lacomis D . Small fiber neuropathy. Muscle and Nerve 2002; 26: 173–188.
Vinik A, Erbas T, Stansberry K, Pittenger G . Small fiber neuropathy and neurovascular disturbances in diabetes mellitus. Exp Clin Endocrinol Diabetes 2001; 109: 451–473.
Singleton J, Smith A, Bromberg M . Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabet Care 2001; 24: 1448–1453.
Brown M, Martin J, Asbury A . Painful diabetic neuropathy. Arch Neurol 1976; 33: 164–171.
Sumner C, Sheth S, Griffin J, Cornblath D, Polydefkis M . The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003; 60: 108–111.
Delaney C, Mouser J, Westerman R . Insulin sensitivity and sensory nerve function in non-diabetic human subjects. Neurosci Lett 1994; 180: 277–280.
Said G, Slama G, Selva J . Progressive centripetal degeneration of axons in small fibre diabetic polyneuropathy: a clinical and pathological study. Brain 1983; 106: 791–807.
Ziegler D, Mayer P, Wiefles K, Gries F . Assessment of small and large fiber function in long-term Type I (Insulin-dependent) diabetic patients with and without painful neuropathy. Pain 1988; 34: 1–10.
Herman R, D'Luzansky S, Gollihare B, Herman J, Juravic M, DiJurio A et al. Hypalgesia and microcirculatory impairment in the forearm of patients with lower limb vasculopathy: small sensory fiber dysfunction. Society for Neuroscience (Abstract) 2000. New Orleans, 731.7.
Lewis T . The Blood Vessels of the Human Skin and Their Responses. Shaw & Sons Ltd.: London, 1927.
Van Hees J, Gybels J . C nociceptor activity in human nerve during painful and non-painful skin stimulation. J Neurol Neurosurg Psychiatry 1981; 44: 600–607.
Magerl W, Treede R . Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol 1996; 497: 837–848.
Holzer P, Maggi C . Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience 1998; 86: 389–398.
Habler H, Wasner G, Janig W . Interaction of sympathetic vasoconstriction and antidromic vasodilatation in the control of skin blood flow. Exp Brain Res 1997; 113: 402–410.
Lewis T . The Nocifensor system of nerves and its reactions. BMJ 1937; 1: 431–435, 491–494.
Willis W, Wu J, Lin Q . Pain and Neuroimmune Interactions. Kluwer Academic/Plenum Publishers: New York, 2000; 99–110.
Holzer P . Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol 1998; 30: 5–11.
D'Luzansky S, Brower J, Herman R, Foley B . Small sensory neuron dysfunction and obesity. Society for Neuroscience (Abstract) 2003. New Orleans, 381.10.
Jancso G, Obal F, Toth-Kasa I, Katona M, Husz S . The modulation of cutaneous inflammatory reactions by peptide containing sensory nerves. Int J Tiss Reac 1985; VII: 449–457.
Szolcsanyi J . A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J Physiol 1977; 73: 251–259.
Caterina M, Julius D . The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci 2001; 24: 487–517.
Morbin M, Capobianco R, Borgna M, Lombardi R, Geppetti P, Davis J et al. Painful diabetic neuropathy (PDN): a morphological study of capsaicin receptor distribution. J Peripher Nerv Syst 2004; 9: 119–120.
Dux M, Sann H, Schemann M, Jancso G . Changes in fibre populations of the rat hairy skin following selective chemodenervation by capsaicin. Cell Tissue Res 1999; 296: 471–477.
Green B, Shaffer G . The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain 1993; 53: 323–334.
Culp W, Ochoa J, Cline M, Dotson R . Heat and mechanical hyperalgesia induced by capsaicin. Brain 1989; 112: 1317–1331.
LaMotte R, Shain C, Simone D, Tsai E-F . Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 1991; 66: 190–211.
Konietzny F, Hensel H . The effect of capsaicin on the response characteristic of human C-Polymodal Nociceptors. J Therm Biol 1983; 8: 213–215.
Schmelz M, Schmidt R, Ringkamp M, Handwerker H, Torebjork H . Sensitization of insensitive branches of c-nociceptors in human skin. J Physiol 1994; 480: 389–394.
Schmelz M, Michael K, Weidner C, Schmidt R, Torebjork H, Handwerker H . Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 1999; 11: 645–648.
Helme R, McKernan S . Neurogenic flare responses following topical application of capsaicin in humans. Ann Neurol 1985; 18: 505–509.
Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork H, Handwerker H . Novel classes of responsive and unresponsive c-nociceptors in human skin. J Neurosci 1995; 15: 333–341.
Holzer P . Capsaicin: cellular targets, mechanism of action, and selectivity for thin-sensory neurons. Pharmacol Rev 1991; 43: 143–201.
Magerl W, Szolcsanyi J, Westerman R, Handwerker H . Laser-doppler measurements of skin vasodilation elicited by percutaneous electrical stimulation of nociceptors in humans. Neurosci Lett 1987; 1982: 349–354.
Wardell K, Naver H, Nilsson G, Wallin B . The cutaneous vascular axon reflex in humans characterized by laser doppler perfusion imaging. J Physiol 1993; 460: 185–199.
Nolano M, Simone D, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy W . Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain 1999; 81: 135–145.
Jaap A, Shore A, Tooke J . Relationship of insulin resistance to microvascular dysfunction in subjects with fasting hyperglycaemia. Diabetologia 1997; 40: 238–243.
Baumann T, Simone D, Shain C, LaMotte R . Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 1991; 66: 212–227.
Rendell M, Bergman T, O'Donnell G, Drobny E, Borgos J, Bonner R . Microvascular blood flow, volume, and velocity measured by laser doppler techniques in IDDM. Diabetes 1989; 38: 819–824.
Cameron N, Cotter M, Archibald V, Dines K, Maxfield E . Anti-oxidant and pro-oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non-diabetic and stretozotocin-diabetic rats. Diabetologia 1994; 37: 449–459.
Carr R, Delaney C, Westerman R, Roberts R . Denervation impairs cutaneous microvascular function and blister healing in the rat hindlimb. Neuroreport 1993; 4: 467–469.
Kramer H, Schmelz M, Berklein F, Bickel A . Electrically stimulated axon reflexes are diminished in diabetic small fiber neuropathies. Diabetes 2004; 53: 769–774.
Khalil Z, Helme R . Sensory peptides as neuromodulators of wound healing in aged rats. J Gerontol 1996; 51A: B354–B361.
Kjartansson J, Dalsgaard C-J, Jonsson C-E . Decreased survival of experimental critical flaps in rats after sensory denervation with capsaicin. Plast Reconstr Surg 1987; 79: 218–221.
Wilson J, Clark J . Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care 2004; 17: 426–435.
Lundeberg T . Peripheral effects of sensory nerve stimulation (acupuncture) in inflammation and ischemia. Scand J Rehab Med Suppl 1993; 29: 61–86.
Heden P, Jernbeck J, Kjartansson J, Samuelson U . Increased skin flap survival and arterial dilation by calcitonin gene-related peptide. Scand J Plast Reconstr Surg 1989; 23: 11–16.
Gherardini G, Gurlek A, Evans G, Milner S, Matarasso A, Wassler M et al. Venous ulcers: improved healing by iontophoretic administration of calcitonin gene-related peptide and vasoactive intestinal polypeptide. Plast Reconstr Surg 1998; 101: 90–93.
Khalil Z, Merhi M . Effects of aging on neurogenic vasodilator responses evoked by transcutaneous electrical nerve stimulation: relevance to wound healing. J Gerontol 2000; 55A: B257–B263.
Lauria G, Holland N, Hauer P, Kornblath D, Griffin J, McArthur J . Epidermal innervation: changes with aging, topographic location, and in sensory neuropathy. J Neurol Sci 1999; 164: 172–178.
Eaglstein W . Wound healing and aging. Clin Geriat Med 1989; 5: 183–188.
Chang D, Wang B, Robb G, Reece G, Miller M, Evans G et al. Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg 2000; 105: 1640–1648.
Acknowledgements
We gratefully thank Alan Newhoff MD and Steven Simon MD for their critical assistance in the recruitment of the morbidly obese subjects. We are also grateful to Mr Stephen D'Luzansky for his early technical assistance. Above all, we appreciate the cooperation of all the subjects who participated in this project. These experiments would not have been possible without the financial assistance of the Banner Good Samaritan Medical Center and the Harrington Department of Bioengineering at Arizona State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herman, R., Brower, J., Stoddard, D. et al. Prevalence of somatic small fiber neuropathy in obesity. Int J Obes 31, 226–235 (2007). https://doi.org/10.1038/sj.ijo.0803418
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803418
Keywords
This article is cited by
-
The Peripheral Neuropathy Prevalence and Characteristics Are Comparable in People with Obesity and Long-Duration Type 1 Diabetes
Advances in Therapy (2022)
-
The role of abnormalities of lipoproteins and HDL functionality in small fibre dysfunction in people with severe obesity
Scientific Reports (2021)
-
Improvements in Diabetic Neuropathy and Nephropathy After Bariatric Surgery: a Prospective Cohort Study
Obesity Surgery (2021)
-
Non-glucose risk factors in the pathogenesis of diabetic peripheral neuropathy
Endocrine (2020)
-
The impact of glycemic variability on diabetic peripheral neuropathy
Endocrine (2016)