Abstract
Aim:
To examine if magnesium lithospermate B (MLB), a potent inhibitor of Na+/K+-ATPase, leads to the elevation of intracellular Ca2+ level as observed in cells treated with cardiac glycosides.
Methods:
Viability of SH-SY5Y neuroblastoma cells treated with various concentrations of ouabain or MLB was measured. Intracellular Ca2+ levels were visualized using Fluo4-AM (fluorescent dye) when cells were treated with ouabain or MLB in the presence or absence of KB-R7943 (Na+/Ca2+ exchanger inhibitor) and 2-APB (IP3 receptor antagonist). Molecular modeling was conducted for the docking of ouabain or MLB to Na+/K+-ATPase. Changes of cell body and dendrite morphology were monitored under a microscope.
Results:
severe toxicity was observed in cells treated with ouabain of concentration higher than 1 μmol/L for 24 h while no apparent toxicity was observed in those treated with MLB. Intracellular Ca2+ levels were substantially elevated by MLB (1 μmol/L) and ouabain (1 μmol/L) in similar patterns, and significantly reduced in the presence of KB-R7943 (10 μmol/L) or 2-APB (100 μmol/L). Equivalent interaction with the binding cavity of Na+/K+-ATPase was simulated for ouabain and MLB by forming five hydrogen bonds, respectively. Treatment of ouabain (1 μmol/L), but not MLB (1 μmol/L), induced dendritic shrink of SH-SY5Y cells.
Conclusion:
Comparable to ouabain, MLB leads to the elevation of intracellular Ca2+ level presumably via the same mechanism by inhibiting Na+/K+-ATPase. The elevated Ca2+ levels seem to be supplied by Ca2+ influx through the reversed mode of the Na+/Ca2+ exchanger and intracellular release from endoplasmic reticulum.
Similar content being viewed by others
Introduction
Gradients of Na+ and K+ across the plasma membrane of animal cells are important for maintaining membrane potentials, cell volume, and active transport of other solutes1. Na+/K+-ATPase, an intrinsic ion transporter on the plasma membrane of animal cells, belongs to the family of P-type cation transporters, and generally consists of a heterodimer of α- and β-subunits2. It pumps 3 Na+ ions out of and 2 K+ ions into the cells at the expense of hydrolyzing one ATP, and thus maintains the gradients of Na+ and K+ ions across the cell membrane. For the continuous exchange of Na+ and K+ across the membrane, Na+/K+-ATPase actively consumes 20%–30% of ATP energy generated in animal cells at rest3.
Cardiac glycosides, such as ouabain and digoxin, are steroid-like compounds and have been used in the treatment of congestive heart failure4. The therapeutic effect of cardiac glycosides lies in their reversible inhibition on the Na+/K+-ATPase of myocardium5. An inhibition on Na+/K+-ATPase leads to the elevation of intracellular Na+ concentration, which in turn activates a Na+/Ca2+exchanger resulting in an increase of intracellular Ca2+ level. The elevated intracellular Ca2+ level induces positive inotropy that eventually accentuates the force of myocardial contraction.
Danshen, the dried roots of medicinal plant Salvia miltiorrhiza, is one of the most popular Chinese herbal products. Traditionally regarded as an effective medicine for the promotion of blood circulation, danshen has been extensively used in the treatment of cardiac and cerebrovascular diseases6. Magnesium lithospermate B (MLB), a derivative of caffeic acid tetramer and the major soluble ingredient in danshen, has been demonstrated to possess several medicinal effects, such as vasodilating, antihypertensive, antioxidative, and free radical scavenging activities7, 8. Recently, MLB was suggested to be responsible for the cardiac therapeutic effect of danshen by its effective inhibition on Na+/K+-ATPase via the same molecular mechanism triggered by cardiac glycosides9. Whether MLB leads to the elevation of intracellular Ca2+ level in cells as observed when they are treated with cardiac glycosides has not been verified.
In this study, SH-SY5Y neuroblastoma cells were employed to examine if MLB treatment may lead to an elevation of intracellular Ca2+ level. Moreover, a Na+/Ca2+ exchanger inhibitor, KB-R7943, and an IP3 receptor antagonist, 2-APB, were utilized to assess possible intracellular and extracellular sources for the fluctuating cytosolic Ca2+ level in SH-SY5Y cells treated with MLB and ouabain. Molecular modeling and docking of ouabain and MLB to Na+/K+-ATPase were exhibited to compare their inhibitory potency at molecular level. Cell viability (toxicity) as well as changes of cell body and dendrite morphology was observed in SH-SY5Y cells.
Materials and methods
Chemicals and reagents
Penicillin, streptomycin, RPMI (Roswell Park Memorial Institute) medium 1640, and calcium free HBSS (Hanks Balanced Salt Solution) buffer were purchased from GIBCO (Grand Island, NY, USA). Ouabain, dimethyl sulfoxide (DMSO) and 2-aminorthyl diphenylborinate (2-APB) were supplied from Sigma-Aldrich (St Louis, MO, USA). Fetal bovine serum (FBS), [4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), fluo-4 acetoxymethyl ester (Fluo4-AM), and 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl] isothiourea methanesulfonate (KB-R7943) were purchased from USB corporation (Cleveland, Ohio, USA), Biological industries (Israel), Molecular Probe (Eugene, Oregon, USA), and Calbiochem (Darmstadt, Germany), respectively. Magnesium lithospermate B (MLB) was extracted from roots of danshen (Salvia miltiorrhiza) and purified as described previously9. Glass bottom culture dishes (35 mm) were obtained from MatTek (Ashland, MA, USA).
Cell cultures
The human adrenergic neuroblastoma cell line, SH-SY5Y10 was kindly provided by Dr Tin-ynu HO of the Graduate Institute of Chinese Medical Science, China Medical University, Taiwan, China. SH-SY5Y cells grown in RPMI-1640 culture medium supplemented with 10% FBS, 1% L-glutamine and 1% penicillin/streptomycin were maintained at 37 °C in a humidified atmosphere of 95% air/5% CO2; and passages were performed every other day by trypsinization. For MTT staining, cells were plated into 24-well culture plates at a density of 1×105 cells. For fluorescence imaging of Ca2+ level and calculation of cell volume, cells were plated in 35 mm glass bottom culture dishes and grown to 80% confluency (approximately 48 h).
Cell viability assay
SH-SY5Y cells treated with ouabain or MLB of concentrations ranging from 10 nmol/L to 100 μmol/L for 5, 10, 30, 60 min, and 24 h were subjected to cell viability assay by MTT staining11. Cells were added with MTT to a final concentration of 500 μg/mL, and incubated at 37 °C for 2 h. After MTT removal, cells were lysed with DMSO. Absorbance was measured using SpectraMax M2 (Molecular Devices, Sunnywale, CA, USA) at a wavelength of 570 nm. Control cells were treated in the same way without adding ouabain or MLB. Viability was expressed in percentage as the absorbance value of cells treated with ouabain or MLB over that of control cells.
Intracellular Ca2+ imaging
Fluctuation of the intracellular Ca2+ level of SH-SY5Y cells was tracked and visualized by a preloaded fluorescent Ca2+-sensitive dye, Fluo4-AM12. Cell-permeable Fluo4-AM was dissolved in DMSO to a concentration of 3 mmol/L, and then further diluted to 3 μmol/L in cell media. The cells were washed once with the culture medium (145 mmol/L NaCl, 5 mmol/L KCl, 2.6 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L HEPES-Na, and 5.6 mmol/L glucose adjusted to pH 7.4 with HCl) and added with the culture medium supplemented with 3 μmol/L Fluo4-AM for 30 min in a humidified 5% CO2 incubator at 37 °C. After washed with the culture medium, cells were added with 1 μmol/L of ouabain or MLB, and Ca2+ fluorescence imaging was monitored at different intervals for 30 min. To examine the possible sources for the elevated cytosolic Ca2+ level in SH-SY5Y cells, 10 μmol/L of KB-R7943 (Na+/Ca2+ exchanger inhibitor) or 100 μmol/L of 2-APB (IP3 receptor antagonist) was added to cells and incubated for 5 or 15 min before the loading of ouabain or MLB in the Ca2+ imaging detection13, 14. KB-R7943 was prepared in ethanol to a concentration of 10 mmol/L, further diluted to 1 mmol/L in Ca2+ free HBSS, and then finally diluted to 10 μmol/L in the culture medium. 2-APB of 100 μmol/L was prepared in the culture medium. For Ca2+ imaging, culture dishes with adherent cells were mounted in the MIU-IBC CO2 incubation system (Olympus, Tokyo, Japan) and placed on the microscope. Time-lapse images of live cells loaded with Fluo4-AM were taken with an UPlanSApo 60×/1.35 oil immersion objective lens, and collected by the Fluoview 1000 confocal scanning microscopy (Olympus, Tokyo, Japan).
Digital image processing
Images collected at different time intervals were processed using the Olympus FV1000 software and NIH ImageJ program (v 1.40) (Bethesda, Maryland, USA). The pictures were acquired at 512×512 pixels, and analyzed frame by frame with a Time Series Analyzer15, 16. This plugin was used to analyze time-lapse image stacks. Each cell was chosen as a region of interest (ROI) through mouse click and its fluorescence intensity of each time point was measured.
Molecular modeling and docking
The crystal structure of shark rectal gland Na+/K+-ATPase (PDB code 3A3Y) was downloaded from Protein Data Bank17. In order to facilitate the docking process, the β and γ subunits of the Na+/K+-ATPase were removed, as well as the water molecules and counter-ions surrounding the remaining α subunit18. After hydrogen saturation, the modified Na+/K+-ATPase was minimized with CHARMm force field19 using the Discover Studio 2.1 package (http://accelrys.com/products/discovery-studio/). The 2D structures of ouabain and MLB were constructed by using the ChemDraw program, and their corresponding 3D structures were converted by the Chem3D program (http://www.cambridgesoft.com/). The binding pocket of the Na+/K+-ATPase α subunit was defined as ouabain occupancy site in the structure of Na+/K+-ATPase-ouabain complex17. Docking of MLB was performed in silico by employing the LigandFit module20 in the Discover Studio 2.1 package. The protein-ligand complexes generated by LigandFit were further minimized with CHARMm forcefield by Smart Minimizer algorithm. Among the candidate structures, reported by the docking simulation, the docking structure with the highest Ligscore1 value, as computed by the score ligand pose module21, was selected to represent MLB inside the binding pocket.
Measurement of relative cell volume
To measure volume of SH-SY5Y cells in different treatments, dishes were mounted in the MIU-IBC CO2 incubation system and placed on the microscope with a differential interference contrast (DIC) mode. The cell profile was viewed through Nomarski optics with a 60× objective (NA, 1.35) under oil immersion and their images were captured consequently. By using the Olympus FV1000 software, ROI was drawn around the cell body or dendrites of each cell. The selected areas were measured. The fractional change in cell volume within each ROI, was expressed as [volume30 min/volume0 min]% where volume0 min and volume30 min represented the cell volume before and after treatment of ouabain or MLB for 30 min, respectively22, 23.
Statistical analysis
Data were expressed as mean±SEM. Relative values for data were compared using analysis of variances (ANOVA) and t-test on SigmaPlot 2001 for Windows version 7.0 (SSI, San Jose, USA). Differences were considered statistically significant at P<0.05.
Results
Effects of MLB and ouabain on viability of SH-SY5Y cells
Viability of SH-SY5Y cells treated with various concentrations (0.01−100 μmol/L) of MLB or ouabain for 5, 10, 30, 60 min, and 24 h was examined (Figure 1). No apparent MLB toxicity to the cells was observed except for a partial reduction of viability when they were treated with 100 μmol/L of this compound for more than 60 min. In contrast, cell viability gradually decreased in a dose-dependent manner when cells were treated with ouabain in the range of 1–100 μmol/L within 60 min, and severe toxicity was observed in cells treated with ouabain of concentration higher than 1 μmol/L for 24 h. On the basis of the above observation, cells were treated with 1 μmol/L of MLB or ouabain for 30 min in the following experiments.
Effects of MLB and ouabain on intracellular Ca2+ levels in SH-SY5Y cells
To examine the effects of MLB and ouabain on their intracellular Ca2+ levels, SH-SY5Y cells were preloaded with Fluo4-AM, incubated with 1 μmol/L of MLB or ouabain, and monitored for their intracellular fluorescence fluctuation at different intervals for 30 min. Compared with cells treated with buffer alone (control), SH-SY5Y cells treated with either MLB or ouabain displayed significantly elevated fluorescence intensity that reached maximum approximately 5-10 min after treatment (Figure 2). These results indicate that MLB and ouabain increased the intracellular Ca2+ levels of SH-SY5Y cells in similar patterns.
Fluctuation of intracellular Ca2+ levels of SH-SY5Y cells treated with MLB and ouabain. SH-SY5Y cells were loaded with Fluo4-AM prior to incubation with 1 μmol/L of MLB or ouabain. Intensity of fluorescence was collected and calculated at different time intervals for 30 min (A). Each point is representative for 40 ROIs of time-lapse images in 5 independent experiments. Serial images of cells treated with MLB and ouabain for 0.5, 7, 15, and 30 min were captured to display the fluctuation of intracellular Ca2+ levels (B). Scale bar represents 20 μm.
Effects of KB-R7943 and 2-APB on the elevation of Ca2+ levels of MLB- and ouabain-treated SH-SY5Y cells
To evaluate the possible calcium sources for the elevation of cytosolic Ca2+ levels in SH-SY5Y cells treated with MLB and ouabain, a Na+/Ca2+ exchanger inhibitor, KB-R7943, or an IP3 receptor antagonist, 2-APB, was added to cells before loading MLB and ouabain. Significant reduction of fluorescence intensity in SH-SY5Y cells by either MLB or ouabain was observed in the presence of KB-R7943 (Figure 3) or 2-APB (Figure 4). These results suggest that the elevated intracellular Ca2+ levels of SH-SY5Y cells treated with either MLB or ouabain were possibly supplied by extracellular Ca2+ influx through the Na+/Ca2+ exchanger on the plasma membrane and intracellular Ca2+ release via the IP3 receptor channel on the membrane of endoplasmic reticulum (ER).
Effects of KB-R7943 on intracellular Ca2+ levels of SH-SY5Y cells elevated by MLB and ouabain. Similar Ca2+ fluorescence imaging was executed as described in Figure 2 except that cells were treated with KB-R7943 for 5 min before the loading of MLB and ouabain. Data represent mean±SEM (n=3).
Effect of 2-APB on intracellular Ca2+ levels of SH-SY5Y cells elevated by MLB and ouabain. Similar Ca2+ fluorescence imaging was executed as described in Figure 2 except that cells were treated with 2-APB for 15 min before the loading of MLB and ouabain. Data represent mean±SEM (n=3).
Docking of MLB to Na+/K+-ATPase
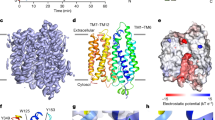
To compare the inhibitory potency of ouabain and MLB on Na+/K+-ATPase at molecular level, both compounds were subjected to molecular modeling and docking to the extracellular domain of Na+/K+-ATPase α subunit. The results showed that MLB could be localized in the ouabain binding pocket of Na+/K+-ATPase, and that equivalent interaction with the binding cavity of Na+/K+-ATPase was observed for ouabain and MLB by forming five intermolecular hydrogen bonds (H-bonds), respectively (Figure 5). Detailed analyses showed that three H-bonds are formed between the lactone of ouabain and Ile328 (forming one H-bond) and Ala330 (forming two H-bonds) of Na+/K+-ATPase, and two H-bonds between the hydroxyl group at C-14 of ouabain and Thr804 of Na+/K+-ATPase. In contrast, three H-bonds are formed between the hydroxyl group at C-4′ position of MLB and Lys912 (forming two H-bonds) and Glu915 (forming one H-bond) of Na+/K+-ATPase, one H-bond between the carbonyl group at C-9' position of MLB and Thr804 of Na+/K+-ATPase, and one H-bond between the hydroxyl group at C-4′′′ position of MLB and Leu110 of Na+/K+-ATPase. Similar to the hydrophobic steroidal core of ouabain, the four aromatic rings of MLB form strong hydrophobic interaction with hydrophobic residues (Leu132, Tyr315, Ile322, Phe323, Ile325, Phe793, Ile794, and Leu802) around the binding pocket of Na+/K+-ATPase.
Detailed molecular interactions between the extracellular binding pocket of Na+/K+-ATPase and ouabain or MLB. (Upper panels) Chemical structures of ouabain and MLB. (Middle panels) Modeling of ouabain and MLB binding to the extracellular pocket of Na+/K+-ATPase α subunit. The amino acid residues around the binding pocket of Na+/K+-ATPase are shown in ribbon structure, and ouabain and MLB in scaled ball and stick. (Lower panels) The amino acid residues of Na+/K+-ATPase close to ouabain or MLB are shown in wireframe, and the structures of ouabain and MLB in scaled ball and stick. Green box or oval represents one or two hydrogen bonds formed between Na+/K+-ATPase and ouabain or MLB.
Effects of MLB or ouabain treatment on cell and dendrite morphology
Changes of cell body and dendrite morphology after treated with 1 μmol/L of MLB or ouabain for 30 min were calculated by measuring the volumes of cell body and dendrites before and after treatment over the same location. Similar to cells of the control group, no significant changes were observed in the volumes of cells treated with MLB or ouabain (Figure 6, left panels). However, a significant shrink of dendrite volume was noticed in cells treated with ouabain, but not in those treated with MLB and those of the control group (Figure 6, right panels). These results suggest that treatment of ouabain, but not MLB, induced dendritic shrink of SH-SY5Y cells under our experimental conditions.
Examination of cell and dendrite volumes after treatment of MLB and ouabain. Changes of cell and dendrite volumes were measured after treated with 1 μmol/L of MLB and ouabain for 30 min. Dendrites indicated by arrows were selected from the examined cells (box areas in left panels) and shown in enlarged photos (right panels). Relative volume (cell or dendrite volume at 30 min over its volume at 0 min) was calculated and shown at the bottom figure. Data represent mean±SEM (n=3). bP<0.05 vs control group. Scale bar represents 20 μm.
Discussion
In a previous study, we demonstrated that MLB could produce potent inhibition of Na+/K+-ATPase in vitro, and proposed that its cardiac therapeutic effects could have been due to the same molecular mechanism as triggered by cardiac glycosides9. In this study, we demonstrated that intracellular Ca2+ levels of SH-SY5Y cells treated with MLB were substantially elevated in a manner similar to that observed in cells treated with ouabain, a cardiac glycoside. Molecular modeling showed that equivalent hydrogen bonding and hydrophobic interaction were observed for ouabain and MLB when these two compounds bound to the cavity of Na+/K+-ATPase, and the results were in agreement with our previous observation that both ouabain and MLB possessed strong inhibitory potency on Na+/K+-ATPase9. Evidently, the results reinforce our previous proposition that MLB, being a potent inhibitor of Na+/K+-ATPase, acts as the active component responsible for the cardiac therapeutic effect of danshen via the same physiological responses subsequently activated by effective inhibition of cardiac glycosides on Na+/K+-ATPase.
Calcium signals are mostly delivered as brief transients that are often organized into regulatory oscillations24. Fluctuation of cytosolic Ca2+ level is generally a coordinated consequence of a number of molecular cascade reactions responsible for Ca2+ influx and efflux in exchange with either extracellular space or intracellular ER storage compartment25. Free Ca2+ enters the cells through either voltage-gated channels or receptor-operated channels located in their plasma membrane26, 27. However, much of the signal Ca2+ comes from the intracellular Ca2+ sources, and is primarily released via the IP3 receptor channel on the ER membrane28, 29. According to the reduction effects of two inhibitors KB-R7943 and 2-APB on the intracellular Ca2+ levels elevated by the treatment of either MLB or ouabain (Figures 3 and 4), the elevation of cytosolic Ca2+ level was likely supplied by both Ca2+ influx through the reversed mode of the Na+/Ca2+ exchanger and intracellular release from ER storage compartment. Whether other sources are also involved in the elevation of intracellular Ca2+ levels in SH-SY5Y cells treated with MLB or ouabain has not been evaluated.
Na+/K+-ATPase is well known for its role as a maintainer of electrolyte and fluid balance in cells, organs and whole body. More and more findings indicate that Na+/K+-ATPase can be a drug target for the treatment of several diseases, including congestive heart failure, ischemic stroke, neurodegenerative diseases and even cancer30, 31, 32. These therapeutic effects may be possibly resulted from the inhibition of Na+/K+-ATPase leading to the fluctuation of Ca2+ level, which in turn activates diverse physiological responses in different cells in variable microenvironments33, 34. However, the inhibition of Na+/K+-ATPase may also lead to the fluctuation of other cations presumably via cross-talks held among different ion channels35, 36. Therefore, the possibility that some pharmacological effects following Na+/K+-ATPase inhibition may be generated by biological reactions insensitive to Ca2+ concentration should not be ruled out.
Dendrite structure is correlated to neuronal function, and its degeneration is generally regarded as an early indicator of cell damage37. In the current study, dendritic shrink was observed in SH-SY5Y cells treated with ouabain, but not in cells treated with MLB (Figure 6); and this observation was in agreement with the toxicity of SH-SY5Y cells treated with high dosages of ouabain, but not with MLB in the viability assay (Figure 1). Similar cell toxicity caused by ouabain at high concentrations has also been noticed in other studies, and the toxicity is blamed to ouabain for its putative triggering of several signaling cascade responses that lead to cell death38. In contrast, MLB is generally regarded as a non-toxic antioxidant, and has also been shown to possess neuroprotective effects against ischemic stroke in a brain slice assay model9. Moreover, water extraction of Salvia miltiorrhiza containing mainly MLB has no toxicity to neonatal rat cardiomyocytes at dosage of 5–80 μg/mL39. Taken together, we surmise that MLB has a great potential, after clinical trials, to become a safer drug than cardiac glycosides.
Author contribution
Ruey-jane FAN and Jason TC TZEN designed research and wrote the paper; Yi-ching CHEN and Tse-yu CHUNG performed research; Tzyy-rong JINN and Feng-yin LI contributed new analytical tools and reagents.
References
Jorgensen PL, Hakansson KO, Karlish SJ . Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu Rev Physiol 2003; 65: 817–49.
Axelsen KB, Palmgren MG . Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol 1998; 46: 84–101.
Skou JC, Esmann M . The Na,K-ATPase. J Bioenerg Biomembr 1992; 24: 249–61.
Li-Saw-Hee FL, Lip GY . Digoxin revisited. QJM 1998; 91: 259–64.
Lin SC, Way EL . A high affinity Ca2+-ATPase enriched nerve-ending plasma membranes. Brain Res 1982; 235: 387–92.
Ji XY, Tan BK, Zhu YZ . Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin 2000; 21: 1089–94.
Jiang RW, Lau KM, Hon PM, Mak TC, Woo KS, Fung KP . Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr Med Chem 2005; 12: 237–46.
Zhao GR, Zhang HM, Ye TX, Xiang ZJ, Yuan YJ, Guo ZX et al. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem Toxicol 2008; 46: 73–81.
Tzen JTC . Jinn TR, Chen YC, Li FY, Cheng FC, Shi LS, et al. Magnesium lithospermate B possesses inhibitory activity on Na+,K+-ATPase and neuroprotective effects against ischemic stroke. Acta Pharmacol Sin 2007; 28: 609–15.
Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS . Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 1978; 38: 3751–7.
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB . Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 1987; 47: 936–42.
Aoshima H, Satoh T, Sakai N, Yamada M, Enokido Y, Ikeuchi T, et al. Generation of free radicals during lipid hydroperoxide-triggered apoptosis in PC12h cells. Biochim Biophys Acta 1997; 1345: 35–42.
Nakamura H, Kawasaki Y, Arakawa N, Saeki M, Maeda S, Koyama Y, et al. The Na+-Ca2+ exchange inhibitor KB-R7943 inhibits high K+-induced increases in intracellular Ca2+ concentration and 3H]noradrenaline release in the human neuroblastoma SH-SY5Y. Neurochem Res 2000; 25: 385–7.
Li XH, Wu YJ . Characteristics of lysophosphatidylcholine-induced Ca2+ response in human neuroblastoma SH-SY5Y cells. Life Sci 2007; 80: 886–92.
Balaji J . http://rsbweb.nih.gov/ij/plugins/time-series.html 2007.
Vicencio JM, Ibarra C, Estrada M, Chiong M, Soto D, Parra V, et al. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology 2006; 147: 1386–95.
Ogawa H, Shinoda T, Cornelius F, Toyoshima C . Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci USA 2009; 106: 13742–7.
Chen RJY . Chung TY, Li FY, Yang WH, Jinn TR, Tzen JTC . Steroid-like compounds in Chinese medicines promote blood circulation via inhibition of Na+/K+-ATPase. Acta Pharmacol Sin 2010; 31: 696–702.
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M . CHARMM: A program for macromolecular energy minimization and dynamics calculations. J Comp Chem 1983; 4: 187–217.
Dixon SL, Merz KM Jr . One-dimensional molecular representations and similarity calculations: methodology and validation. J Med Chem 2001; 44: 3795–809.
Krammer A, Kirchhoff PD, Jiang X, Venkatachalam CM, Waldman M . LigScore: a novel scoring function for predicting binding affinities. J Mol Graph Model 2005; 23: 395–407.
Takeuchi S, Ando M, Irimajiri A . Changes in the volume of marginal cells induced by isotonic 'Cl– depletion/restoration': involvement of the Cl– channel and Na+-K+-Cl– cotransporter. Hear Res 1997; 113: 99–109.
Clough AV, Haworth ST, Ma W, Dawson CA . Effects of hypoxia on pulmonary microvascular volume. Am J Physiol Heart Circ Physiol 2000; 279: H1274–82.
Berridge MJ . Calcium microdomains: organization and function. Cell Calcium 2006; 40: 405–12.
Verkhratsky A, Orkand RK, Kettenmann H . Glial calcium: homeostasis and signaling function. Physiol Rev 1998; 78: 99–141.
Pearce B, Albrecht J, Morrow C, Murphy S . Astrocyte glutamate receptor activation promotes inositol phospholipid turnover and calcium flux. Neurosci Lett 1986; 72: 335–40.
MacVicar BA, Hochman D, Delay MJ, Weiss S . Modulation of intracellular Ca++ in cultured astrocytes by influx through voltage-activated Ca++ channels. Glia 1991; 4: 448–55.
Kostyuk P, Verkhratsky A . Calcium stores in neurons and glia. Neuroscience 1994; 63: 381–404.
Deitmer JW, Verkhratsky AJ, Lohr C . Calcium signalling in glial cells. Cell Calcium 1998; 24: 405–16.
Xie Z, Xie J . The Na+/K+-ATPase-mediated signal transduction as a target for new drug development. Front Biosci 2005; 10: 3100–9.
Mijatovic T, Ingrassia L, Facchini V, Kiss R . Na+/K+-ATPase alpha subunits as new targets in anticancer therapy. Expert Opin Ther Targets 2008; 12: 1403–17.
Prassas I, Diamandis EP . Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov 2008; 7: 926–35.
Wang JK, Portbury S, Thomas MB, Barney S, Ricca DJ, Morris DL, et al. Cardiac glycosides provide neuroprotection against ischemic stroke: discovery by a brain slice-based compound screening platform. Proc Natl Acad Sci USA 2006; 103: 10461–6.
Tzen JTC . Chen RJY, Chung TY, Chen YC, Lin NH . Active compounds in Chinese herbs and medicinal animal products for promoting blood circulation via inhibition of Na+,K+-ATPase. Chang Gung Med J 2010; 33:126–36.
Hasegawa H . Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci 2004; 95: 153–7.
Chen RJY . Chung TY, Li FY, Lin NH, Icitsnwe JTC . Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin 2009; 30: 61–9.
Bywood PT, Johnson SM . Dendrite loss is a characteristic early indicator of toxin-induced neurodegeneration in rat midbrain slices. Exp Neurol 2000; 161: 306–16.
Kulikov A, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G. Ouabain activates signaling pathways associated with cell death in human neuroblastoma. Biochim Biophys Acta 2007; 1768: 1691–702.
Ou-yang X, Takahashi K, Komatsu K, Nakamura N, Hattori M, Baba A, et al. Protective effect of Salvia miltiorrhiza on angiotensin II-induced hypertrophic responses in neonatal rat cardiac cells. Jpn J Pharmacol 2001; 87: 289–96.
Acknowledgements
Project supported by a grant to Jason TC TZEN from the National Science Council, Taiwan, China (No 96-2752-B-005-008-PAE).
We thank Prof Chih-ning SUN (Department of Entomology, National Chuang Hsing University) for critical reading of the manuscript, Ms Ya-chin HOU in our laboratory for the assistance in the cell viability assay, and the Core Facility of Graduate Institute of Biotechnology, National Chuang Hsing University for providing the confocal microscope.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, Yc., Jinn, Tr., Chung, Ty. et al. Magnesium lithospermate B extracted from Salvia miltiorrhiza elevats intracellular Ca2+ level in SH-SY5Y cells. Acta Pharmacol Sin 31, 923–929 (2010). https://doi.org/10.1038/aps.2010.102
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.102
Keywords
This article is cited by
-
Neurological disorders of COVID-19: insights to applications of natural products from plants and microorganisms
Archives of Pharmacal Research (2022)
-
Detecting metabolites of different transition metallithospermate B complexes after intravenous injection in rats
Acta Pharmacologica Sinica (2014)
-
Enhancing the potency of lithospermate B for inhibiting Na+/K+-ATPase activity by forming transition metal ion complexes
Acta Pharmacologica Sinica (2013)
-
Active ingredients in Chinese medicines promoting blood circulation as Na+/K+-ATPase inhibitors
Acta Pharmacologica Sinica (2011)