Abstract
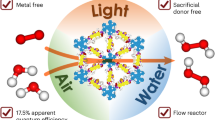
Covalent organic frameworks (COFs) with ordered π skeletons and aligned nanopores could be ideal photocatalytic materials but remain unexplored for this use. Here we report hexavalent photocatalytic COFs for efficient photosynthesis via systematic design of the π skeletons and pores. The framework of the photocatalysts have donor-alt-acceptor arrangements and upon irradiation are converted into catalytic scaffolds, which have dense catalytic sites for oxygen reduction and water oxidation and spatially segregated donor and acceptor columns for hole and electron separation to prevent charge recombination and enable rapid charge transport. The pore walls are engineered to be hydrophilic to enable water and dissolved oxygen to pass through the one-dimensional channel to reach the catalytic sites via capillary effect. The COFs act as a photocatalyst for the photosynthesis of H2O2 using only water, air and light, attaining a high production rate of 7.2 mmol g–1 h–1, optimal apparent quantum yield of 18.0% and solar-to-chemical conversion efficiency of 0.91% in bath reactors. Flow reactors incorporating these COFs can continuously produce pure H2O2 solution, yielding over 15 litres under ambient conditions, and exhibit exceptional operational stability over 2 weeks of use.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Source Data are provided with this paper. All other data supporting the finding of this study are available within this article and its Supplementary Information. The atomic coordinates for the final optimized structures are provided as Supplementary Data 1–4.
References
Shaegh, S. A. M., Nguyen, N.-T., Ehteshami, S. M. M. & Chan, S. H. A membraneless hydrogen peroxide fuel cell using Prussian blue as cathode material. Energy Environ. Sci. 5, 8225–8228 (2012).
Fukuzumi, S., Yamada, Y. & Karlin, K. D. Hydrogen peroxide as a sustainable energy carrier: electrocatalytic production of hydrogen peroxide and the fuel cell. Electrochim. Acta 82, 493–511 (2012).
Mase, K., Yoneda, M., Yamada, Y. & Fukuzumi, S. Seawater usable for production and consumption of hydrogen peroxide as a solar fuel. Nat. Commun. 7, 11470 (2016).
Yamada, Y., Fukunishi, Y., Yamazaki, S.-i & Fukuzumi, S. Hydrogen peroxide as sustainable fuel: electrocatalysts for production with a solar cell and decomposition with a fuel cell. Chem. Comm. 46, 7334–7336 (2010).
Wen, Y. et al. Electrochemical reactors for continuous decentralized H2O2 production. Angew. Chem. Int. Ed. 61, e202205972 (2022).
Chang, J.-N. et al. Oxidation–reduction molecular junction covalent organic frameworks for full reaction photosynthesis of H2O2. Angew. Chem. Int. Ed. 62, e202218868 (2023).
Zhi, Q. et al. Piperazine-linked metalphthalocyanine frameworks for highly efficient visible-light-driven H2O2 photosynthesis. J. Am. Chem. Soc. 144, 21328–21336 (2022).
Hou, H., Zeng, X. & Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 59, 17356–17376 (2020).
Shiraishi, Y. et al. Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 18, 985–993 (2019).
Liu, L. et al. Linear conjugated polymers for solar-driven hydrogen peroxide production: the importance of catalyst stability. J. Am. Chem. Soc. 143, 19287–19293 (2021).
Teng, Z. Y. et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 4, 374–384 (2021).
Wu, C. B. et al. Polarization engineering of covalent triazine frameworks for highly efficient photosynthesis of hydrogen peroxide from molecular oxygen and water. Adv. Mater. 34, adma.202110266 (2022).
Kou, M. P. et al. Molecularly engineered covalent organic frameworks for hydrogen peroxide photosynthesis. Angew. Chem. Int. Ed. 61, e.202200413 (2022).
Chu, C. H. et al. Spatially separating redox centers on 2D carbon nitride with cobalt single atom for photocatalytic H2O2 production. Proc. Natl Acad. Sci. USA 117, 6376–6382 (2020).
Krishnaraj, C. et al. Strongly reducing (diarylamino)benzene-based covalent organic framework for metal-free visible light photocatalytic H2O2 generation. J. Am. Chem. Soc. 142, 20107–20116 (2020).
Chen, D. et al. Covalent organic frameworks containing dual O2 reduction centers for overall photosynthetic hydrogen peroxide production. Angew. Chem. Int. Ed. 62, e202217479 (2023).
Chen, L. et al. Acetylene and diacetylene functionalized covalent triazine frameworks as metal-free photocatalysts for hydrogen peroxide production: a new two-electron water oxidation pathway. Adv. Mater. 32, 1904433 (2020).
Zeng, X. et al. Simultaneously tuning charge separation and oxygen reduction pathway on graphitic carbon nitride by polyethylenimine for boosted photocatalytic hydrogen peroxide production. ACS Catal. 10, 3697–3706 (2020).
Segura, J. L., Mancheño, M. J. & Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: synthesis, properties and potential applications. Chem. Soc. Rev. 45, 5635–5671 (2016).
Wang, Z., Zhang, S., Chen, Y., Zhang, Z. & Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 49, 708–735 (2020).
Haase, F. & Lotsch, B. V. Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 49, 8469–8500 (2020).
Wang, G.-B. et al. Covalent organic frameworks: emerging high-performance platforms for efficient photocatalytic applications. J. Mater. Chem. A 8, 6957–6983 (2020).
Wang, H. et al. Covalent organic framework photocatalysts: structures and applications. Chem. Soc. Rev. 49, 4135–4165 (2020).
Ascherl, L. et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 8, 310–316 (2016).
Xu, X. et al. Pore partition in two-dimensional covalent organic frameworks. Nat. Commun. 14, 3360 (2023).
Xu, X., Cai, P., Chen, H., Zhou, H.-C. & Huang, N. Three-dimensional covalent organic frameworks with she topology. J. Am. Chem. Soc. 144, 18511–18517 (2022).
Gui, B. et al. Crystallization of dimensional isomers in covalent organic frameworks. J. Am. Chem. Soc. 145, 11276–11281 (2023).
Gui, B. et al. Tailoring the pore surface of 3D covalent organic frameworks via post-synthetic click chemistry. Angew. Chem. Int. Ed. 61, e202113852 (2022).
Liu, R. et al. Covalent organic frameworks: an ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 50, 120–242 (2021).
He, T. et al. Integrated interfacial design of covalent organic framework photocatalysts to promote hydrogen evolution from water. Nat. Commun. 14, 329 (2023).
Tao, S. & Jiang, D. Covalent organic frameworks for energy conversions: current status, challenges, and perspectives. CCS Chem. 3, 2003–2024 (2021).
He, T., Geng, K. & Jiang, D. Engineering covalent organic frameworks for light-driven hydrogen production from water. ACS Materials Lett. 1, 203–208 (2019).
Tan, K. T. et al. Covalent organic frameworks. Nat. Rev. Methods Prime. 3, 1–19 (2023).
Deng, L., Ding, Z., Ye, X. & Jiang, D. Covalent organic frameworks: chemistry of pore interface and wall surface perturbation and impact on functions. Acc. Mater. Res. 3, 879–893 (2022).
Jiang, D. Covalent organic frameworks: an amazing chemistry platform for designing polymers. Chem 6, 2461–2483 (2020).
Feng, S. et al. Bicarbazole-based redox-active covalent organic frameworks for ultrahigh-performance energy storage. Chem. Comm. 53, 11334–11337 (2017).
Wang, H., Yang, C., Chen, F., Zheng, G. & Han, Q. A crystalline partially fluorinated triazine covalent organic framework for efficient photosynthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 61, e202202328 (2022).
Sun, J. et al. Metal–organic frameworks and covalent organic frameworks as photocatalysts for H2O2 production from oxygen and water. J. Mater. Chem. A 11, 21516–21540 (2023).
Deng, M. et al. Extending the π-conjugation system of covalent organic frameworks for more efficient photocatalytic H2O2 production. Green Chem. 25, 3069–3076 (2023).
Chen, H., Jena, H. S., Feng, X., Leus, K. & Van Der Voort, P. Engineering covalent organic frameworks as heterogeneous photocatalysts for organic transformations. Angew. Chem. Int. Ed. 61, e202204938 (2022).
Wang, L. et al. Understanding photocatalytic hydrogen peroxide production in pure water for benzothiadiazole-based covalent organic frameworks. Catal. Sci. Technol. 13, 6463–6471 (2023).
Cheng, H. et al. Rational design of covalent heptazine frameworks with spatially separated redox centers for high‐efficiency photocatalytic hydrogen peroxide production. Adv. Mater. 34, 2107480 (2022).
Zhai, L. et al. Constructing synergistic triazine and acetylene cores in fully conjugated covalent organic frameworks for cascade photocatalytic H2O2 production. Chem. Mater. 34, 5232–5240 (2022).
Chang, J.-N. et al. Regulation of redox molecular junctions in covalent organic frameworks for H2O2 photosynthesis coupled with biomass valorization. Angew. Chem. Int. Ed. 62, e202303606 (2023).
Yong, Z. & Ma, T. Solar-to-H2O2 catalyzed by covalent organic frameworks. Angew. Chem. Int. Ed. 62, e202308980 (2023).
Liu, R. et al. Linkage-engineered donor–acceptor covalent organic frameworks for optimal photosynthesis of hydrogen peroxide from water and air. Nat. Catal. 7, 195–206 (2024).
Sun, J. et al. Pyrene-based covalent organic frameworks for photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 62, e202216719 (2023).
He, T. & Zhao, Y. Covalent organic frameworks for energy conversion in photocatalysis. Angew. Chem. Int. Ed. 62, e202303086 (2023).
Jin, E. et al. Exceptional electron conduction in two-dimensional covalent organic frameworks. Chem 7, 3309–3324 (2021).
He, T. et al. Bottom-up interfacial design of covalent organic frameworks for highly efficient and selective electrocatalysis of CO2. Adv. Mater. 34, 2205186 (2022).
Tan, K. T., Tao, S., Huang, N. & Jiang, D. Water cluster in hydrophobic crystalline porous covalent organic frameworks. Nat. Commun. 12, 6747 (2021).
Sun, C. et al. 2D covalent organic framework for water harvesting with fast kinetics and low regeneration temperature. Angew. Chem. Int. Ed. 62, e202217103 (2023).
Li, J. et al. Covalent organic frameworks: reversible 3D coalesce via interlocked skeleton–pore actions and impacts on π electronic structures. J. Am. Chem. Soc. 145, 26383–26392 (2023).
Bunck, D. N. & Dichtel, W. R. Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 135, 14952–14955 (2013).
Stegbauer, L., Schwinghammer, K. & Lotsch, B. V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 5, 2789–2793 (2014).
Briega-Martos, V., Cheuquepán, W. & Feliu, J. M. Detection of superoxide anion oxygen reduction reaction intermediate on Pt(111) by infrared reflection absorption spectroscopy in neutral pH conditions. J. Phys. Chem. Lett. 12, 1588–1592 (2021).
Smith, B. C. Fundamentals of Fourier Transform Infrared Spectroscopy (CRC Press, 2011).
Berthomieu, C. & Hienerwadel, R. Fourier transform infrared (FTIR) spectroscopy. Photosynth. Res. 101, 157–170 (2009).
Voraberger, H., Ribitsch, V., Janotta, M. & Mizaikoff, B. Application of mid-infrared spectroscopy: measuring hydrogen peroxide concentrations in bleaching baths. Appl. Spectrosc. 57, 574–579 (2003).
Giguère, P. A. The infra‐red spectrum of hydrogen peroxide. J. Chem. Phys. 18, 88–92 (1950).
Żegli´nski, J., Piotrowski, G. P. & Pięks´, R. A study of interaction between hydrogen peroxide and silica gel by FTIR spectroscopy and quantum chemistry. J. Mol. Struct. 794, 83–91 (2006).
Acknowledgements
D.J. gratefully acknowledges funding from Singapore MOE Tier 2 grants (T2EP10220-0004 and T2EP10221-0012), Singapore MOE Tier 1 grants (A-0008368-00-00 and A-0008369-00-00) and a Singapore A*STAR grant (U2102d2004). T.C.S. gratefully acknowledges funding from Singapore MOE Tier 2 grants (MOE2019-T2-1-097 and MOE-T2EP50120-0004) and a National Research Foundation Singapore NRF Investigatorship (NRF-NRFI-2018-04). We thank J.W. for the use of a flow pump, N.Y. for DRIFTS, L.Q. for the measurement of contact angle and C.X. for providing the g-C3N4 sample.
Author information
Authors and Affiliations
Contributions
D.J. conceived the idea and led the project. Y.C. and R.L. conducted the experiments and measurements. Y.G. and T.C.S. conducted the transient absorption measurements. G.W. and W.Y. performed the calculations. Y.C., R.L., Y.G., G.W., T.C.S., S.W.Y. and D.J. interpreted the results and Y.C. and D.J. wrote the paper. All authors have read and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Peer review
Peer review information
Nature Synthesis thanks Pascal Van Der Voort and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Electrostatic potential distribution.
a–c, Electrostatic potential maps for TP-DPBD1O-COF (a), TP-DPBD2O-COF (b) and TP-DPBD3O-COF (c).
Extended Data Fig. 2 Photocatalytic pathway.
a, Active site of diphenylbutadiyne linker for oxygen reduction reaction. b, Photocatalytic cycle for oxygen reduction reaction over butadiyne unit of the linker. c, Active site for water oxidation reaction at the triphenylene knot. d, Photocatalytic cycle for water oxidation over the phenyl unit of the knot.
Supplementary Information
Supplementary Information
Materials and methods, synthetic procedures, Supplementary Tables 1–6 and Figs. 1–60.
Supplementary Video
Supplementary Videos 1–4
Supplementary Data 1
Refined crystal structure of TP-DPBD1O-COF.
Supplementary Data 2
Refined crystal structure of TP-DPBD1O-COF.
Supplementary Data 3
Refined crystal structure of TP-DPBD3O-COF.
Supplementary Data 4
Refined crystal structure of TP-QPh-COF.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Liu, R., Guo, Y. et al. Hierarchical assembly of donor–acceptor covalent organic frameworks for photosynthesis of hydrogen peroxide from water and air. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00542-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00542-4